Dopamine D1 and D5 receptors are localized to discrete populations of interneurons in primate prefrontal cortex
- PMID: 19020206
- PMCID: PMC2705695
- DOI: 10.1093/cercor/bhn212
Dopamine D1 and D5 receptors are localized to discrete populations of interneurons in primate prefrontal cortex
Abstract
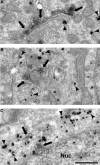
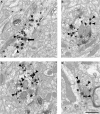
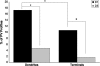
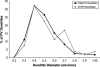
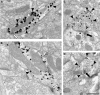
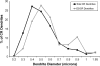
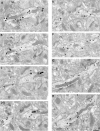
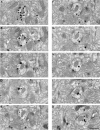
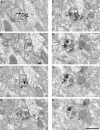
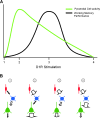
Working memory (WM) is a core cognitive process that depends upon activation of D1 family receptors (D1R) and inhibitory interneurons in the prefrontal cortex (PFC). D1R are comprised of the D(1) and D(5) subtypes, and D(5) has a 10-fold higher affinity for dopamine. Parvalbumin (PV) and calretinin (CR) are 2 interneuron populations that are differentially affected by D1R stimulation and have discrete postsynaptic targets, such that PV interneurons provide strong inhibition to pyramidal cells, whereas CR interneurons inhibit other interneurons. The distinct properties of both the D1R and interneuron subtypes may contribute to the "inverted-U" relationship of D1R stimulation and WM ability. To determine the prevalence of D(1) and D(5) in PV and CR interneurons, we performed quantitative double-label immunoelectron microscopy in layer III of macaque area 9. We found that D(1) was the predominant D1R subtype in PV interneurons and was found mainly in dendrites. In contrast, D(5) was the predominant D1R subtype in CR interneurons and was found mainly in dendrites. Integrating these findings with previously published electrophysiological data, we propose a circuitry model as a framework for understanding the inverted-U relationship between dopamine stimulation of D1R and WM performance.
Figures



Similar articles
-
D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization.J Neurosci. 1998 Dec 15;18(24):10553-65. doi: 10.1523/JNEUROSCI.18-24-10553.1998. J Neurosci. 1998. PMID: 9852592 Free PMC article.
-
Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: coexpression in dendritic spines and axon terminals.J Comp Neurol. 2008 Jun 20;508(6):893-905. doi: 10.1002/cne.21710. J Comp Neurol. 2008. PMID: 18399540 Free PMC article.
-
Stress Impairs Prefrontal Cortical Function via D1 Dopamine Receptor Interactions With Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels.Biol Psychiatry. 2015 Dec 15;78(12):860-70. doi: 10.1016/j.biopsych.2015.01.009. Epub 2015 Feb 4. Biol Psychiatry. 2015. PMID: 25731884 Free PMC article.
-
Dopamine's Actions in Primate Prefrontal Cortex: Challenges for Treating Cognitive Disorders.Pharmacol Rev. 2015 Jul;67(3):681-96. doi: 10.1124/pr.115.010512. Pharmacol Rev. 2015. PMID: 26106146 Free PMC article. Review.
-
Mapping pathologic circuitry in schizophrenia.Handb Clin Neurol. 2018;150:389-417. doi: 10.1016/B978-0-444-63639-3.00025-6. Handb Clin Neurol. 2018. PMID: 29496154 Review.
Cited by
-
Reciprocal Alterations in Regulator of G Protein Signaling 4 and microRNA16 in Schizophrenia.Schizophr Bull. 2016 Mar;42(2):396-405. doi: 10.1093/schbul/sbv139. Epub 2015 Sep 30. Schizophr Bull. 2016. PMID: 26424323 Free PMC article.
-
Mesocorticolimbic Dopamine Pathways Across Adolescence: Diversity in Development.Front Neural Circuits. 2021 Sep 8;15:735625. doi: 10.3389/fncir.2021.735625. eCollection 2021. Front Neural Circuits. 2021. PMID: 34566584 Free PMC article. Review.
-
The Emotional Gatekeeper: A Computational Model of Attentional Selection and Suppression through the Pathway from the Amygdala to the Inhibitory Thalamic Reticular Nucleus.PLoS Comput Biol. 2016 Feb 1;12(2):e1004722. doi: 10.1371/journal.pcbi.1004722. eCollection 2016 Feb. PLoS Comput Biol. 2016. PMID: 26828203 Free PMC article.
-
Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination.Nature. 2015 Oct 15;526(7573):448-52. doi: 10.1038/nature14957. Epub 2015 Sep 30. Nature. 2015. PMID: 26416758 Free PMC article.
-
Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT.Neuropsychopharmacology. 2010 Jul;35(8):1708-17. doi: 10.1038/npp.2010.35. Epub 2010 Mar 31. Neuropsychopharmacology. 2010. PMID: 20357758 Free PMC article.
References
-
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. - PubMed
-
- Celio MR. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231:995–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

