Premutation allele pool in myotonic dystrophy type 2
- PMID: 19020295
- PMCID: PMC2677510
- DOI: 10.1212/01.wnl.0000333665.01888.33
Premutation allele pool in myotonic dystrophy type 2
Abstract
Background: The myotonic dystrophies (DM1, DM2) are the most common adult muscle diseases and are characterized by multisystem involvement. DM1 has been described in diverse populations, whereas DM2 seems to occur primarily in European Caucasians. Both are caused by the expression of expanded microsatellite repeats. In DM1, there is a reservoir of premutation alleles; however, there have been no reported premutation alleles for DM2. The (CCTG)(DM2) expansion is part of a complex polymorphic repeat tract of the form (TG)(n)(TCTG)(n)(CCTG)(n)(NCTG)(n)(CCTG)(n). Expansions are as large as 40 kb, with the expanded (CCTG)(n) motif uninterrupted. Reported normal alleles have up to (CCTG)(26) with one or more interruptions.
Methods: To identify and characterize potential DM2 premutation alleles, we cloned and sequenced 43 alleles from 23 individuals. Uninterrupted alleles were identified, and their instability was confirmed by small-pool PCR. We determined the genotype of a nearby single nucleotide polymorphism (rs1871922) known to be in linkage disequilibrium with the DM2 mutation.
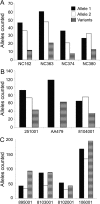
Results: We identified three classes of large non-DM2 repeat alleles: 1) up to (CCTG)(24) with two interruptions, 2) up to (CCTG)(32) with up to four interruptions, and 3) uninterrupted (CCTG)(22-33). Large non-DM2 alleles were more common in African Americans than in European Caucasians. Uninterrupted alleles were significantly more unstable than interrupted alleles (p = 10(-4) to 10(-7)). Genotypes at rs1871922 were consistent with the hypothesis that all large alleles occur on the same haplotype as the DM2 expansion.
Conclusions: We conclude that unstable uninterrupted (CCTG)(22-33) alleles represent a premutation allele pool for DM2 full mutations.
Figures


Comment in
-
How much expansion to be diseased?: toward repeat size and myotonic dystrophy type 2.Neurology. 2009 Feb 10;72(6):484-5. doi: 10.1212/01.wnl.0000341937.70150.64. Neurology. 2009. PMID: 19204257 No abstract available.
Similar articles
-
Myotonic dystrophy type 2: human founder haplotype and evolutionary conservation of the repeat tract.Am J Hum Genet. 2003 Oct;73(4):849-62. doi: 10.1086/378720. Epub 2003 Sep 22. Am J Hum Genet. 2003. PMID: 14505273 Free PMC article.
-
Uninterrupted CCTG tracts in the myotonic dystrophy type 2 associated locus.Neuromuscul Disord. 2013 Jul;23(7):591-8. doi: 10.1016/j.nmd.2013.02.013. Epub 2013 Apr 2. Neuromuscul Disord. 2013. PMID: 23561036
-
Distribution and Structure of DM2 Repeat Tract Alleles in the German Population.Front Neurol. 2018 Jun 19;9:463. doi: 10.3389/fneur.2018.00463. eCollection 2018. Front Neurol. 2018. PMID: 29973908 Free PMC article.
-
Myotonic dystrophy: emerging mechanisms for DM1 and DM2.Biochim Biophys Acta. 2007 Feb;1772(2):195-204. doi: 10.1016/j.bbadis.2006.05.013. Epub 2006 Jun 20. Biochim Biophys Acta. 2007. PMID: 16876389 Review.
-
Myotonic dystrophies.Chang Gung Med J. 2005 Aug;28(8):517-26. Chang Gung Med J. 2005. PMID: 16265841 Review.
Cited by
-
Short tandem repeat expansions in sporadic amyotrophic lateral sclerosis and frontotemporal dementia.Sci Adv. 2023 May 5;9(18):eade2044. doi: 10.1126/sciadv.ade2044. Epub 2023 May 5. Sci Adv. 2023. PMID: 37146135 Free PMC article.
-
(CCUG)n RNA toxicity in a Drosophila model of myotonic dystrophy type 2 (DM2) activates apoptosis.Dis Model Mech. 2017 Aug 1;10(8):993-1003. doi: 10.1242/dmm.026179. Epub 2017 Jun 16. Dis Model Mech. 2017. PMID: 28623239 Free PMC article.
-
If you build a rare disease registry, will they enroll and will they use it? Methods and data from the National Registry of Myotonic Dystrophy (DM) and Facioscapulohumeral Muscular Dystrophy (FSHD).Contemp Clin Trials. 2012 Mar;33(2):302-11. doi: 10.1016/j.cct.2011.11.016. Epub 2011 Nov 26. Contemp Clin Trials. 2012. PMID: 22155025 Free PMC article.
-
Proximal myopathy: causes and associated conditions.Discoveries (Craiova). 2022 Dec 31;10(4):e160. doi: 10.15190/d.2022.19. eCollection 2022 Oct-Dec. Discoveries (Craiova). 2022. PMID: 37483534 Free PMC article. Review.
-
Overexpression of CUGBP1 in skeletal muscle from adult classic myotonic dystrophy type 1 but not from myotonic dystrophy type 2.PLoS One. 2013 Dec 20;8(12):e83777. doi: 10.1371/journal.pone.0083777. eCollection 2013. PLoS One. 2013. PMID: 24376746 Free PMC article.
References
-
- Harper PS. Myotonic Dystrophy, 3rd ed. London, UK: W.B. Saunders; 2001.
-
- Krahe R, Bachinski LL, Udd B. Myotonic dystrophy type 2: clinical and genetic aspects. In: Wells RD, Ashizawa T, eds. Genetic Instabilities and Neurological Diseases. Amsterdam, Boston: Academic Press/Elsevier; 2006:131–150.
-
- Brook JD, et al. Correction: Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992;69:385. - PubMed
-
- Fu YH, Pizzuti A, Fenwick RG Jr, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 1992;255:1256–1258. - PubMed
-
- Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 1992;255:1253–1255. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
