c-Myc is required for maintenance of glioma cancer stem cells
- PMID: 19020659
- PMCID: PMC2582454
- DOI: 10.1371/journal.pone.0003769
c-Myc is required for maintenance of glioma cancer stem cells
Abstract
Background: Malignant gliomas rank among the most lethal cancers. Gliomas display a striking cellular heterogeneity with a hierarchy of differentiation states. Recent studies support the existence of cancer stem cells in gliomas that are functionally defined by their capacity for extensive self-renewal and formation of secondary tumors that phenocopy the original tumors. As the c-Myc oncoprotein has recognized roles in normal stem cell biology, we hypothesized that c-Myc may contribute to cancer stem cell biology as these cells share characteristics with normal stem cells.
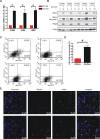
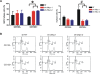
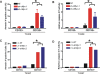
Methodology/principal findings: Based on previous methods that we and others have employed, tumor cell populations were enriched or depleted for cancer stem cells using the stem cell marker CD133 (Prominin-1). We characterized c-Myc expression in matched tumor cell populations using real time PCR, immunoblotting, immunofluorescence and flow cytometry. Here we report that c-Myc is highly expressed in glioma cancer stem cells relative to non-stem glioma cells. To interrogate the significance of c-Myc expression in glioma cancer stem cells, we targeted its expression using lentivirally transduced short hairpin RNA (shRNA). Knockdown of c-Myc in glioma cancer stem cells reduced proliferation with concomitant cell cycle arrest in the G(0)/G(1) phase and increased apoptosis. Non-stem glioma cells displayed limited dependence on c-Myc expression for survival and proliferation. Further, glioma cancer stem cells with decreased c-Myc levels failed to form neurospheres in vitro or tumors when xenotransplanted into the brains of immunocompromised mice.
Conclusions/significance: These findings support a central role of c-Myc in regulating proliferation and survival of glioma cancer stem cells. Targeting core stem cell pathways may offer improved therapeutic approaches for advanced cancers.
Conflict of interest statement
Figures
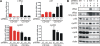


Similar articles
-
Targeting cancer stem cells through L1CAM suppresses glioma growth.Cancer Res. 2008 Aug 1;68(15):6043-8. doi: 10.1158/0008-5472.CAN-08-1079. Cancer Res. 2008. PMID: 18676824 Free PMC article.
-
Brain cancer stem cells display preferential sensitivity to Akt inhibition.Stem Cells. 2008 Dec;26(12):3027-36. doi: 10.1634/stemcells.2007-1073. Epub 2008 Sep 18. Stem Cells. 2008. PMID: 18802038 Free PMC article.
-
Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro.BMC Cancer. 2008 Oct 22;8:304. doi: 10.1186/1471-2407-8-304. BMC Cancer. 2008. PMID: 18940013 Free PMC article.
-
Biology of glioma cancer stem cells.Mol Cells. 2009 Jul 31;28(1):7-12. doi: 10.1007/s10059-009-0111-2. Epub 2009 Jul 20. Mol Cells. 2009. PMID: 19655094 Review.
-
CD133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme.Neurosurg Clin N Am. 2012 Jul;23(3):391-405. doi: 10.1016/j.nec.2012.04.011. Epub 2012 Jun 5. Neurosurg Clin N Am. 2012. PMID: 22748652 Review.
Cited by
-
Long non-coding RNAs and MYC association in hematological malignancies.Ann Hematol. 2020 Oct;99(10):2231-2242. doi: 10.1007/s00277-020-04166-4. Epub 2020 Jul 4. Ann Hematol. 2020. PMID: 32621182 Review.
-
Novel function of MDA-9/Syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells.Oncotarget. 2016 Aug 23;7(34):54102-54119. doi: 10.18632/oncotarget.10851. Oncotarget. 2016. PMID: 27472461 Free PMC article.
-
ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma.PLoS One. 2012;7(6):e38842. doi: 10.1371/journal.pone.0038842. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22761708 Free PMC article.
-
Extracellular Vesicles Released by Glioblastoma Cells Stimulate Normal Astrocytes to Acquire a Tumor-Supportive Phenotype Via p53 and MYC Signaling Pathways.Mol Neurobiol. 2019 Jun;56(6):4566-4581. doi: 10.1007/s12035-018-1385-1. Epub 2018 Oct 23. Mol Neurobiol. 2019. PMID: 30353492 Free PMC article.
-
Interplay between NRF1, E2F4 and MYC transcription factors regulating common target genes contributes to cancer development and progression.Cell Oncol (Dordr). 2018 Oct;41(5):465-484. doi: 10.1007/s13402-018-0395-3. Epub 2018 Jul 25. Cell Oncol (Dordr). 2018. PMID: 30047092 Review.
References
-
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. - PubMed
-
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. - PubMed
-
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. - PubMed
-
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

