Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis
- PMID: 19020666
- PMCID: PMC2582490
- DOI: 10.1371/journal.pone.0003779
Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis
Abstract
Background: The maturation of Plasmodium falciparum gametocytes in the human host takes several days, during which the parasites need to efficiently evade the host immune system. Like asexual stage parasites, immature gametocytes can sequester at various sites in the human body, and only mature sexual stages are found in the circulation. Although the fundamental mechanisms of gametocyte immune evasion are still largely unknown, candidate molecules that may be involved include variant antigens encoded by multigene families in the P. falciparum genome, such as the PfEMP1, STEVOR and RIFIN proteins. While expression of the former two families in sexual stages has been investigated earlier, we report here RIFIN expression during gametocytogenesis.
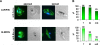
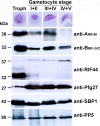
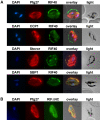
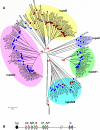
Methodology/principal findings: Variants of two previously characterized RIFIN subfamilies (A- and B-type RIFINs) were found to be synthesized in gametocytes. Immunofluorescence experiments showed A-type RIFINs to be accumulated in a crescent-shaped pattern of discrete punctate structures at the infected erythrocyte membrane, while members of the B-type family were associated with the parasite. Transcription analysis demonstrated the existence of diverse transcriptional regulation patterns during sexual differentiation and indicated variant-specific regulation of B-type RIFINs, in contrast to group-specific regulation for A-type RIFINs. Phylogenetic analysis of 5'-upstream regions showed that the rif-gene family falls into five defined clusters, designated rups (rifupstream) A1, A2, AB, B and C. In trophozoites and early gametocytes, rif variants of the rupsA2-type were preferentially expressed.
Conclusions/significance: In this work we demonstrate the expression dynamics of the rif-gene family during sexual differentiation and present indications for subgroup specific regulation patterns. Therefore, our data provide a first foundation and point to new directions for future investigations of the potential role of RIFINs in gametocyte immune evasion.
Conflict of interest statement
Figures



Similar articles
-
Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes.Eukaryot Cell. 2006 Aug;5(8):1206-14. doi: 10.1128/EC.00029-06. Eukaryot Cell. 2006. PMID: 16896206 Free PMC article.
-
Identification of a major rif transcript common to gametocytes and sporozoites of Plasmodium falciparum.Malar J. 2010 May 28;9:147. doi: 10.1186/1475-2875-9-147. Malar J. 2010. PMID: 20509952 Free PMC article.
-
Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns.Mol Biochem Parasitol. 2007 Nov;156(1):51-61. doi: 10.1016/j.molbiopara.2007.07.011. Epub 2007 Jul 21. Mol Biochem Parasitol. 2007. PMID: 17719658
-
Variant surface antigens of Plasmodium falciparum and their roles in severe malaria.Nat Rev Microbiol. 2017 Aug;15(8):479-491. doi: 10.1038/nrmicro.2017.47. Epub 2017 Jun 12. Nat Rev Microbiol. 2017. PMID: 28603279 Review.
-
Transcription and coregulation of multigene families in Plasmodium falciparum.Trends Parasitol. 2007 May;23(5):183-6; discussion 186-7. doi: 10.1016/j.pt.2007.02.010. Epub 2007 Mar 9. Trends Parasitol. 2007. PMID: 17350338 Review.
Cited by
-
Plasmodium helical interspersed subtelomeric family-an enigmatic piece of the Plasmodium biology puzzle.Parasitol Res. 2019 Oct;118(10):2753-2766. doi: 10.1007/s00436-019-06420-9. Epub 2019 Aug 15. Parasitol Res. 2019. PMID: 31418110 Review.
-
Chromatin-mediated epigenetic regulation in the malaria parasite Plasmodium falciparum.Eukaryot Cell. 2010 Aug;9(8):1138-49. doi: 10.1128/EC.00036-10. Epub 2010 May 7. Eukaryot Cell. 2010. PMID: 20453074 Free PMC article. Review.
-
Long- and short-term selective forces on malaria parasite genomes.PLoS Genet. 2010 Sep 9;6(9):e1001099. doi: 10.1371/journal.pgen.1001099. PLoS Genet. 2010. PMID: 20838588 Free PMC article.
-
Genomics and integrated systems biology in Plasmodium falciparum: a path to malaria control and eradication.Parasite Immunol. 2012 Feb-Mar;34(2-3):50-60. doi: 10.1111/j.1365-3024.2011.01340.x. Parasite Immunol. 2012. PMID: 21995286 Free PMC article. Review.
-
Analysis of subtelomeric virulence gene families in Plasmodium falciparum by comparative transcriptional profiling.Mol Microbiol. 2012 Apr;84(2):243-59. doi: 10.1111/j.1365-2958.2012.08019.x. Epub 2012 Mar 22. Mol Microbiol. 2012. PMID: 22435676 Free PMC article.
References
-
- WHO. 2005 World Malaria Report.
-
- Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134:1911–1929. - PubMed
-
- Sauerwein RW. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect. 2007;9:792–795. - PubMed
-
- Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol. 2007;154:119–123. - PubMed
-
- Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

