Imaging performance of a miniature integrated microendoscope
- PMID: 19021400
- PMCID: PMC3129652
- DOI: 10.1117/1.2978060
Imaging performance of a miniature integrated microendoscope
Abstract
An integrated miniature multi-modal microscope (4M device) for microendoscopy was built and tested. Imaging performance is evaluated and imaging results are presented for both fluorescence and reflectance samples. Images of biological samples show successful imaging of both thin layers of fixed cells prepared on a slide as well as thick samples of excised fixed porcine epithelial tissue, thus demonstrating the potential for in vivo use.
Figures




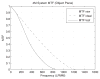



References
-
- American Cancer Society. Cancer Facts and Figures, 2006. 2006 http://cancer.org.
-
- Neil MAA, Juskaitis R, Wilson T. Method of obtaining optical sectioning by using structured light in a conventional microscope. Opt Lett. 1997;22:1905–1907. - PubMed
-
- Gmitro A, Aziz D. Confocal microscopy through a fiber-optic imaging bundle. Opt Lett. 1993;18:565–567. - PubMed
-
- Delaney P, Harris M, King R. Fibre-optic laser scanning confocal microscope suitable for fluorescence imaging. Appl Opt. 1994;33:573–577. - PubMed
-
- Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle P, Neurath M. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

