The yeast Coq4 polypeptide organizes a mitochondrial protein complex essential for coenzyme Q biosynthesis
- PMID: 19022396
- PMCID: PMC2627766
- DOI: 10.1016/j.bbalip.2008.10.006
The yeast Coq4 polypeptide organizes a mitochondrial protein complex essential for coenzyme Q biosynthesis
Abstract
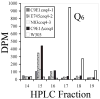
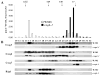
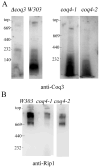
Coenzyme Q is a redox active lipid essential for aerobic respiration. The Coq4 polypeptide is required for Q biosynthesis and growth on non-fermentable carbon sources, however its exact function in this pathway is not known. Here we probe the functional roles of Coq4p in a yeast Q biosynthetic polypeptide complex. A yeast coq4-1 mutant harboring an E226K substitution is unable to grow on nonfermentable carbon sources. The coq4-1 yeast mutant retains significant Coq3p O-methyltransferase activity, and mitochondria isolated from coq4-1 and coq4-2 (E(121)K) yeast point mutants contain normal steady state levels of Coq polypeptides, unlike the decreased levels of Coq polypeptides generally found in strains harboring coq gene deletions. Digitonin-solubilized mitochondrial extracts prepared from yeast coq4 point mutants show that Coq3p and Coq4 polypeptides no longer co-migrate as high molecular mass complexes by one- and two-dimensional Blue Native-PAGE. Similarly, gel filtration chromatography confirms that O-methyltransferase activity, Coq3p, Coq4p, and Coq7p migration are disorganized in the coq4-1 mutant mitochondria. The data suggest that Coq4p plays an essential role in organizing a Coq enzyme complex required for Q biosynthesis.
Figures



References
-
- Crane FL. Distribution of Quinones. In: Morton RA, editor. Biochemistry of Quinones. Vol. 1. Academic Press; London: 1965. pp. 183–206.
-
- Debray FG, Lambert M, Mitchell GA. Disorders of mitochondrial function. Curr Opin Pediatr. 2008;20:471–482. - PubMed
-
- Galpern WR, Cudkowicz ME. Coenzyme Q treatment of neurodegenerative diseases of aging. Mitochondrion. 2007;7(Suppl):S146–153. - PubMed
-
- Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(Suppl):S154–167. - PubMed
-
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

