Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model
- PMID: 19022495
- PMCID: PMC2787105
- DOI: 10.1016/j.jaci.2008.10.011
Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model
Abstract
Background: Food allergy is a disorder in which antigenic food proteins elicit immune responses. Animal models of food allergy have several limitations that influence their utility, including failure to recapitulate several key immunologic hallmarks. Consequently, little is known regarding the pathogenesis and mechanisms leading to food allergy. Staphylococcus aureus-derived enterotoxins, a common cause of food contamination, are associated with antigen responses in atopic dermatitis.
Objective: We hypothesized that S aureus-derived enterotoxins might influence the development of food allergy. We examined the influence of administration of staphylococcal enterotoxin B (SEB) with food allergens on immunologic responses and compared these responses with those elicited by a cholera toxin-driven food allergy model.
Methods: Oral administration of ovalbumin or whole peanut extract with or without SEB was performed once weekly. After 8 weeks, mice were challenged with oral antigen alone, and the physiologic and immunologic responses to antigen were studied.
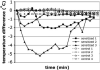
Results: SEB administered with antigen resulted in immune responses to the antigen. Responses were highly T(H)2 polarized, and oral challenge with antigen triggered anaphylaxis and local and systemic mast cell degranulation. SEB-driven sensitization induced eosinophilia in the blood and intestinal tissues not observed with cholera toxin sensitization. SEB impaired tolerance specifically by impairing expression of TGF-beta and regulatory T cells, and tolerance was restored with high-dose antigen.
Conclusions: We demonstrate a new model of food allergy to oral antigen in common laboratory strains of mice that recapitulates many features of clinical food allergy that are not seen in other models. We demonstrate that SEB impairs oral tolerance and permits allergic responses.
Figures





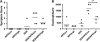
References
-
- Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral administration of IL-12 suppresses anaphylactic reactions in a murine model of peanut hypersensitivity. Clin Immunol. 2001;101:220–8. - PubMed
-
- Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108:639–46. - PubMed
-
- Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171–8. - PubMed
-
- van Wijk F, Hartgring S, Koppelman SJ, Pieters R, Knippels LM. Mixed antibody and T cell responses to peanut and the peanut allergens Ara h 1, Ara h 2, Ara h 3 and Ara h 6 in an oral sensitization model. Clin Exp Allergy. 2004;34:1422–8. - PubMed
-
- van Wijk F, Hoeks S, Nierkens S, Koppelman SJ, van Kooten P, Boon L, et al. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J Immunol. 2005;174:174–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

