Reprogramming and differentiation in mammals: motifs and mechanisms
- PMID: 19022741
- PMCID: PMC2735112
- DOI: 10.1101/sqb.2008.73.016
Reprogramming and differentiation in mammals: motifs and mechanisms
Abstract
The natural reprogramming of the mammalian egg and sperm genomes is an efficient process that takes place in less than 24 hours and gives rise to a totipotent zygote. Transfer of somatic nuclei to mammalian oocytes also leads to their reprogramming and formation of totipotent embryos, albeit very inefficiently and requiring an activation step. Reprogramming of differentiated cells to induced pluripotent stem (iPS) cells takes place during a period of time substantially longer than reprogramming of the egg and sperm nuclei and is significantly less efficient. The stochastic expression of endogenous proteins during this process would imply that controlled expression of specific proteins is crucial for reprogramming to take place. The fact that OCT4, NANOG, and SOX2 form the core components of the pluripotency circuitry would imply that control at the transcriptional level is important for reprogramming to iPS cells. In contradistinction, the much more efficient reprogramming of the mammalian egg and sperm genomes implies that other levels of control are necessary, such as chromatin remodeling, translational regulation, and efficient degradation of no longer needed proteins and RNAs.
Figures

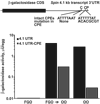
 Graph of reporter construct containing the intact CPE sequence;
Graph of reporter construct containing the intact CPE sequence;  Graph of reporter construct containing the mutated CPE sequence.
Graph of reporter construct containing the mutated CPE sequence.
Similar articles
-
Embryonic stem cell potency fluctuates with endogenous retrovirus activity.Nature. 2012 Jul 5;487(7405):57-63. doi: 10.1038/nature11244. Nature. 2012. PMID: 22722858 Free PMC article.
-
Establishment of totipotency does not depend on Oct4A.Nat Cell Biol. 2013 Sep;15(9):1089-97. doi: 10.1038/ncb2816. Epub 2013 Aug 11. Nat Cell Biol. 2013. PMID: 23934214 Free PMC article.
-
Trib2 regulates the pluripotency of embryonic stem cells and enhances reprogramming efficiency.Exp Mol Med. 2017 Nov 24;49(11):e401. doi: 10.1038/emm.2017.191. Exp Mol Med. 2017. PMID: 29170476 Free PMC article.
-
Role of Oct4 in maintaining and regaining stem cell pluripotency.Stem Cell Res Ther. 2010 Dec 14;1(5):39. doi: 10.1186/scrt39. Stem Cell Res Ther. 2010. PMID: 21156086 Free PMC article. Review.
-
Totipotency, pluripotency and nuclear reprogramming.Adv Biochem Eng Biotechnol. 2009;114:185-99. doi: 10.1007/10_2008_45. Adv Biochem Eng Biotechnol. 2009. PMID: 19343304 Free PMC article. Review.
Cited by
-
Tackling Societal Challenges Related to Ageing and Transport Transition: An Introduction to Philosophical Principles of Causation Adapted to the Biopsychosocial Model.Geriatrics (Basel). 2015 Dec 23;1(1):3. doi: 10.3390/geriatrics1010003. Geriatrics (Basel). 2015. PMID: 31022799 Free PMC article. Review.
-
Myoblast-derived neuronal cells form glutamatergic neurons in the mouse cerebellum.Stem Cells. 2010 Oct;28(10):1839-47. doi: 10.1002/stem.509. Stem Cells. 2010. PMID: 20799335 Free PMC article.
-
DNA replication is an integral part of the mouse oocyte's reprogramming machinery.PLoS One. 2014 May 16;9(5):e97199. doi: 10.1371/journal.pone.0097199. eCollection 2014. PLoS One. 2014. PMID: 24836291 Free PMC article.
-
Friend or Foe: Epigenetic Regulation of Retrotransposons in Mammalian Oogenesis and Early Development.Yale J Biol Med. 2016 Dec 23;89(4):487-497. eCollection 2016 Dec. Yale J Biol Med. 2016. PMID: 28018140 Free PMC article. Review.
-
Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos.Sci Rep. 2017 Aug 15;7(1):8299. doi: 10.1038/s41598-017-08266-6. Sci Rep. 2017. PMID: 28811525 Free PMC article.
References
-
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo AB, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RD, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O'Brien CM, Oh SK, Olsson C, Otonkoski T, Park KY, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RA, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, van den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803. - PubMed
-
- Belloc E, Mendez R. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature. 2008;452:1017. - PubMed
-
- Bevilacqua A, Kinnunen LH, Mangia F. Genetic manipulation of mammalian dictyate oocytes: factors affecting transient expression of microinjected DNA templates. Mol Reprod Dev. 1992;33:124. - PubMed
-
- Bushati N, Stark A, Brennecke J, Cohen SM. Temporal reciprocity of miRNAs and their targets during the maternal-to-zygotic transition in Drosophila. Curr Biol. 2008;18:501. - PubMed
-
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
