Initiation and elongation in fibrillation of ALS-linked superoxide dismutase
- PMID: 19022905
- PMCID: PMC2585484
- DOI: 10.1073/pnas.0807058105
Initiation and elongation in fibrillation of ALS-linked superoxide dismutase
Abstract
Familial amyotrophic lateral sclerosis (fALS) caused by mutations in copper-zinc superoxide dismutase (SOD1) is characterized by the presence of SOD1-rich inclusions in spinal cords. Similar inclusions observed in fALS transgenic mice have a fibrillar appearance suggestive of amyloid structure. Metal-free apo-SOD1 is a relatively stable protein and has been shown to form amyloid fibers in vitro only when it has been subjected to severely destabilizing conditions, such as low pH or reduction of its disulfide bonds. Here, by contrast, we show that a small amount of disulfide-reduced apo-SOD1 can rapidly initiate fibrillation of this exceptionally stable and highly structured protein under mild, physiologically accessible conditions, thus providing an unusual demonstration of a specific, physiologically relevant form of a protein acting as an initiating agent for the fibrillation of another form of the same protein. We also show that, once initiated, elongation can proceed via recruitment of either apo- or partially metallated disulfide-intact SOD1 and that the presence of copper, but not zinc, ions inhibits fibrillation. Our findings provide a rare glimpse into the specific changes in a protein that can lead to nucleation and into the ability of amyloid nuclei to recruit diverse forms of the same protein into fibrils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
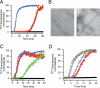

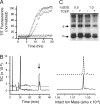
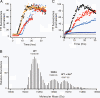
Comment in
-
ALS precursor finally shaken into fibrils.Proc Natl Acad Sci U S A. 2008 Dec 2;105(48):18649-50. doi: 10.1073/pnas.0810568106. Epub 2008 Nov 24. Proc Natl Acad Sci U S A. 2008. PMID: 19033195 Free PMC article. No abstract available.
References
-
- Valentine JS, Doucette PA, Potter SZ. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. - PubMed
-
- Tiwari A, Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol Chem. 2003;278:5984–5992. - PubMed
-
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. - PubMed
-
- Shibata N, et al. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1994;179:149–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

