Docosahexaenoic acid and the aging brain
- PMID: 19022980
- PMCID: PMC2666388
- DOI: 10.3945/jn.108.096016
Docosahexaenoic acid and the aging brain
Abstract
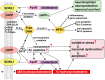
The dietary essential PUFA docosahexaenoic acid [DHA; 22:6(n-3)] is a critical contributor to cell structure and function in the nervous system, and deficits in DHA abundance are associated with cognitive decline during aging and in neurodegenerative disease. Recent studies underscore the importance of DHA-derived neuroprotectin D1 (NPD1) in the homeostatic regulation of brain cell survival and repair involving neurotrophic, antiapoptotic and antiinflammatory signaling. Emerging evidence suggests that NPD1 synthesis is activated by growth factors and neurotrophins. Evolving research indicates that NPD1 has important determinant and regulatory interactions with the molecular-genetic mechanisms affecting beta-amyloid precursor protein (betaAPP) and amyloid beta (Abeta) peptide neurobiology. Deficits in DHA or its peroxidation appear to contribute to inflammatory signaling, apoptosis, and neuronal dysfunction in Alzheimer disease (AD), a common and progressive age-related neurological disorder unique to structures and processes of the human brain. This article briefly reviews our current understanding of the interactions of DHA and NPD1 on betaAPP processing and Abeta peptide signaling and how this contributes to oxidative and pathogenic processes characteristic of aging and AD pathology.
Figures
References
-
- Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function. Annu Rev Cell Dev Biol. 2005;21:633–57. - PubMed
-
- Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9. - PubMed
-
- Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci. 2001;16:159–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

