GIT1 mediates VEGF-induced podosome formation in endothelial cells: critical role for PLCgamma
- PMID: 19023093
- PMCID: PMC2735351
- DOI: 10.1161/ATVBAHA.108.174391
GIT1 mediates VEGF-induced podosome formation in endothelial cells: critical role for PLCgamma
Abstract
Objective: We and others showed that tyrosine kinase receptors (TKRs) such as the epidermal growth factor receptor stimulate G protein-coupled receptor (GPCR) kinase-interacting protein 1 (GIT1) phosphorylation via c-Src, which is required for phospholipase C-gamma (PLCgamma) activation, indicating that GIT1 participates in TKR signaling. VEGF is the most important TKR in endothelial cells (ECs); essential for cell survival, migration, and angiogenesis. Podosomes, actin-rich structures, were found to contribute to EC migration, tissue invasion, and matrix remodeling, suggesting a role for podosomes in angiogenesis. Because GIT1 is a substrate of c-Src, and podosome formation is c-Src dependent, we hypothesized that GIT1 plays an important role in VEGF-induced EC podosome formation and cell migration.
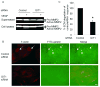
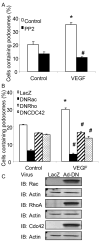
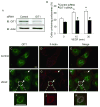
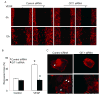
Methods and results: Exposure of ECs to VEGF for 30 minutes stimulated GIT1 colocalization with podosomes. Depletion of GIT1 by siRNA significantly decreased VEGF-induced podosome formation. A key role for PLCgamma was suggested by several experiments. Double staining PLCgamma and actin showed colocalization of PLCgamma with podosomes. Podosome formation was dramatically reduced by PLCgamma inhibitor U73122, Src inhibitor PP2, or expression of dominant negative small GTPases. Therefore, VEGF-induced EC podosome formation is dependent on Src, GIT1, PLCgamma, and small GTPases. In addition, matrix metalloprotease 2 (MMP2) and MT-MMP1 were detected at sites of VEGF-induced podosomes. Depletion of GIT1 by siRNA also significantly inhibited VEGF-induced MMP2 activation and extracellular matrix (ECM) degradation. Therefore, GIT1 mediates VEGF-induced matrix metalloproteinase (MMP) activation and ECM degradation by regulating podosome formation. Finally, depletion of GIT1 by siRNA significantly decreased VEGF-induced cell migration.
Conclusions: These data indicate that GIT1 is an essential mediator for VEGF-induced EC podosome formation and cell migration via PLCgamma.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

