A critical role of a cellular membrane traffic protein in poliovirus RNA replication
- PMID: 19023417
- PMCID: PMC2581890
- DOI: 10.1371/journal.ppat.1000216
A critical role of a cellular membrane traffic protein in poliovirus RNA replication
Abstract
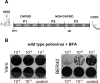
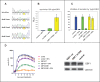
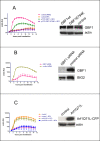
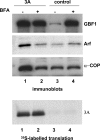
Replication of many RNA viruses is accompanied by extensive remodeling of intracellular membranes. In poliovirus-infected cells, ER and Golgi stacks disappear, while new clusters of vesicle-like structures form sites for viral RNA synthesis. Virus replication is inhibited by brefeldin A (BFA), implicating some components(s) of the cellular secretory pathway in virus growth. Formation of characteristic vesicles induced by expression of viral proteins was not inhibited by BFA, but they were functionally deficient. GBF1, a guanine nucleotide exchange factor for the small cellular GTPases, Arf, is responsible for the sensitivity of virus infection to BFA, and is required for virus replication. Knockdown of GBF1 expression inhibited virus replication, which was rescued by catalytically active protein with an intact N-terminal sequence. We identified a mutation in GBF1 that allows growth of poliovirus in the presence of BFA. Interaction between GBF1 and viral protein 3A determined the outcome of infection in the presence of BFA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
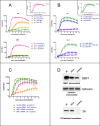
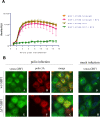
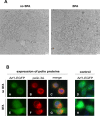

References
-
- Bienz K, Egger D, Rasser Y, Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983;131:39–48. - PubMed
-
- Caliguiri LA, Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969;166:885–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

