Mouse hematopoietic stem cell identification and analysis
- PMID: 19023891
- PMCID: PMC2640229
- DOI: 10.1002/cyto.a.20674
Mouse hematopoietic stem cell identification and analysis
Abstract
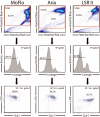
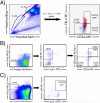
Hematopoietic stem cells (HSCs) remain by far the most well-characterized adult stem cell population both in terms of markers for purification and assays to assess functional potential. However, despite over 40 years of research, working with HSCs in the mouse remains difficult because of the relative abundance (or lack thereof) of these cells in the bone marrow. The frequency of HSCs in bone marrow is about 0.01% of total nucleated cells and approximately 5,000 can be isolated from an individual mouse depending on the age, sex, and strain of mice as well as purification scheme utilized. This prohibits the study of processes in HSCs, which require large amounts of starting material. Adding to the challenge is the continual reporting of new markers for HSC purification, which makes it difficult for the uninitiated in the field to know which purification strategies yield the highest proportion of long-term, multilineage HSCs. This report will review different hematopoietic stem and progenitor purification strategies and compare flow cytometry profiles for HSC sorting and analysis on different instruments. We will also discuss methods for rapid flow cytometric analysis of peripheral blood cell types, and novel strategies for working with rare cell populations such as HSCs in the analysis of cell cycle status by BrdU, Ki-67, and Pyronin Y staining. The purpose of this review is to provide insight into some of the recent experimental and technical advances in mouse hematopoietic stem cell biology.
Figures





References
-
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. - PubMed
-
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

