Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields
- PMID: 19025913
- PMCID: PMC2750002
- DOI: 10.1002/mrm.21790
Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields
Abstract
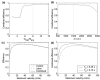
Continuous labeling by flow-driven adiabatic inversion is advantageous for arterial spin labeling (ASL) perfusion studies, but details of the implementation, including inefficiency, magnetization transfer, and limited support for continuous-mode operation on clinical scanners, have restricted the benefits of this approach. Here a new approach to continuous labeling that employs rapidly repeated gradient and radio frequency (RF) pulses to achieve continuous labeling with high efficiency is characterized. The theoretical underpinnings, numerical simulations, and in vivo implementation of this pulsed continuous ASL (PCASL) method are described. In vivo PCASL labeling efficiency of 96% relative to continuous labeling with comparable labeling parameters far exceeded the 33% duty cycle of the PCASL RF pulses. Imaging at 3T with body coil transmission was readily achieved. This technique should help to realize the benefits of continuous labeling in clinical imagers.
(c) 2008 Wiley-Liss, Inc.
Figures
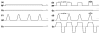








References
-
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. - PubMed
-
- Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30(2):115–124. - PubMed
-
- Aguirre GK, Detre JA, Wang J. Perfusion fMRI for functional neuroimaging. Int Rev Neurobiol. 2005;66:213–236. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

