Differentiation and transplantation of human embryonic stem cell-derived hepatocytes
- PMID: 19026649
- PMCID: PMC2732349
- DOI: 10.1053/j.gastro.2008.10.047
Differentiation and transplantation of human embryonic stem cell-derived hepatocytes
Abstract
Background & aims: The ability to obtain unlimited numbers of human hepatocytes would improve the development of cell-based therapies for liver diseases, facilitate the study of liver biology, and improve the early stages of drug discovery. Embryonic stem cells are pluripotent, potentially can differentiate into any cell type, and therefore could be developed as a source of human hepatocytes.
Methods: To generate human hepatocytes, human embryonic stem cells were differentiated by sequential culture in fibroblast growth factor 2 and human activin-A, hepatocyte growth factor, and dexamethasone. Functional hepatocytes were isolated by sorting for surface asialoglycoprotein-receptor expression. Characterization was performed by real-time polymerase chain reaction, immunohistochemistry, immunoblot, functional assays, and transplantation.
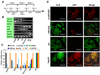
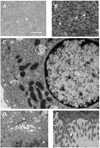
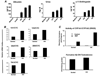
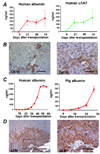
Results: Embryonic stem cell-derived hepatocytes expressed liver-specific genes, but not genes representing other lineages, secreted functional human liver-specific proteins similar to those of primary human hepatocytes, and showed human hepatocyte cytochrome P450 metabolic activity. Serum from rodents given injections of embryonic stem cell-derived hepatocytes contained significant amounts of human albumin and alpha1-antitrypsin. Colonies of cytokeratin-18 and human albumin-expressing cells were present in the livers of recipient animals.
Conclusions: Human embryonic stem cells can be differentiated into cells with many characteristics of primary human hepatocytes. Hepatocyte-like cells can be enriched and recovered based on asialoglycoprotein-receptor expression and potentially could be used in drug discovery research and developed as therapeutics.
Conflict of interest statement
Conflicts of interest: There are no conflicts of interest to disclose.
Figures


Comment in
-
Cell transplantation to replace whole liver transplantation.Gastroenterology. 2009 Mar;136(3):767-9. doi: 10.1053/j.gastro.2009.01.019. Epub 2009 Jan 22. Gastroenterology. 2009. PMID: 19167399 No abstract available.
References
-
- Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192:892–894. - PubMed
-
- Groth CG, Arborgh B, Bjorken C, Sundberg B, Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977;9:313–316. - PubMed
-
- Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40:878–886. - PubMed
-
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

