Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis
- PMID: 19027740
- PMCID: PMC2712351
- DOI: 10.1053/j.gastro.2008.10.081
Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis
Abstract
Background & aims: Inflammatory bowel disease (IBD) is a multifactorial disease thought to be caused by alterations in epithelial function, innate and adaptive immunity, and luminal microbiota. The specific role of epithelial barrier function remains undefined, although increased activity of intestinal epithelial myosin light chain kinase (MLCK), which is the primary mechanism of tumor necrosis factor-induced barrier dysfunction, occurs in human IBD. Our aim was to determine whether, in an intact epithelium, primary dysregulation of the intestinal epithelial barrier by pathophysiologically relevant mechanisms can contribute to development of colitis.
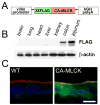
Methods: We developed transgenic (Tg) mice that express constitutively active MLCK (CA-MLCK) specifically within intestinal epithelia. Their physiology, immune status, and susceptibility to disease were assessed and compared with non-Tg littermate controls.
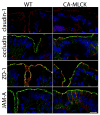
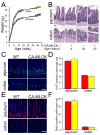
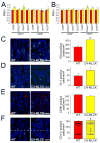
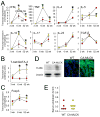
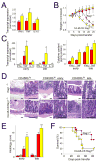
Results: CA-MLCK Tg mice demonstrated significant barrier loss but grew and gained weight normally and did not develop spontaneous disease. CA-MLCK Tg mice did, however, develop mucosal immune activation demonstrated by increased numbers of lamina propria CD4(+)lymphocytes, redistribution of CD11c+cells, increased production of interferon-gamma and tumor necrosis factor, as well as increased expression of epithelial major histocompatibility complex class I. When challenged with CD4+CD45+Rb(hi) lymphocytes, Tg mice developed an accelerated and more severe form of colitis and had shorter survival times than non-Tg littermates.
Conclusions: Primary pathophysiologically relevant intestinal epithelial barrier dysfunction is insufficient to cause experimental intestinal disease but can broadly activate mucosal immune responses and accelerate the onset and severity of immune-mediated colitis.
Conflict of interest statement
No conflicts of interest exist
Figures

References
-
- Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–5. - PubMed
-
- May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–32. - PubMed
-
- Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology. 1989;97:927–31. - PubMed
-
- Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 DK094954/DK/NIDDK NIH HHS/United States
- P30CA14599/CA/NCI NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- T32 HL007237/HL/NHLBI NIH HHS/United States
- R01 DK061931/DK/NIDDK NIH HHS/United States
- R01 DK068271/DK/NIDDK NIH HHS/United States
- R01DK61931/DK/NIDDK NIH HHS/United States
- R01DK77905/DK/NIDDK NIH HHS/United States
- R01 DK077905/DK/NIDDK NIH HHS/United States
- P01 DK067887/DK/NIDDK NIH HHS/United States
- P01DK67887/DK/NIDDK NIH HHS/United States
- R01DK68271/DK/NIDDK NIH HHS/United States
- T32GM07281/GM/NIGMS NIH HHS/United States
- T32 GM007281/GM/NIGMS NIH HHS/United States
- T32HL007237/HL/NHLBI NIH HHS/United States
- R01 DK058964/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

