Susceptibility contrast in high field MRI of human brain as a function of tissue iron content
- PMID: 19027861
- PMCID: PMC2670442
- DOI: 10.1016/j.neuroimage.2008.10.029
Susceptibility contrast in high field MRI of human brain as a function of tissue iron content
Erratum in
- Neuroimage. 2012 Sep;62(3):2173
Abstract
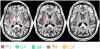
Magnetic susceptibility provides an important contrast mechanism for MRI. Increasingly, susceptibility-based contrast is being exploited to investigate brain tissue microstructure and to detect abnormal levels of brain iron as these have been implicated in a variety of neuro-degenerative diseases. However, it remains unclear to what extent magnetic susceptibility-related contrast at high field relates to actual brain iron concentrations. In this study, we performed susceptibility weighted imaging as a function of field strength on healthy brains in vivo and post-mortem brain tissues at 1.5 T, 3 T and 7 T. Iron histology was performed on the tissue samples for comparison. The calculated susceptibility-related parameters R(2)(*) and signal frequency shift in four iron-rich regions (putamen, globus pallidus, caudate, and thalamus) showed an almost linear dependence (r>or=0.90 for R(2)(*); r>or=0.83 for phase, p<0.01) on field strength, suggesting that potential ferritin saturation effects are not relevant to susceptibility-weighted contrast for field strengths up to 7 T. The R(2)(*) dependence on the putative (literature-based) iron concentration was 0.048 Hz/T/ppm. The histological data from brain samples confirmed the linear dependence of R(2)(*) on field strength and showed a slope against iron concentration of 0.0099 Hz/T/ppm dry-weight, which is equivalent to 0.05 Hz/T/ppm wet-weight and closely matched the calculated value in vivo. These results confirm the validity of using susceptibility-weighted contrast as an indicator of iron content in iron-rich brain regions. The absence of saturation effects opens the way to exploit the benefits of MRI at high field strengths for the detection of iron distributions with high sensitivity and resolution.
Figures




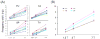



References
-
- Abduljalil AM, Schmalbrock P, Novak V, Chakeres DW. Enhanced gray and white matter contrast of phase susceptibility-weighted images in ultra-high-field magnetic resonance imaging. J Magn Reson Imaging. 2003;18:284–290. - PubMed
-
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European Heart Journal. 2001;22:2171–2179. - PubMed
-
- Bartzokis G, Sultzer D, Mintz J, Holt LE, Marx P, Phelan CK, Marder SR. In vivo evaluation of brain iron in Alzheimer’s disease and normal subjects using MRI. Biol Psychiatry. 1994;35:480–487. - PubMed
-
- Bartzokis G, Tishler TA. MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimer’s and Huntingon’s disease. Cell Mol Biol (Noisy-le-grand) 2000;46:821–833. - PubMed
-
- Bizzi A, Brooks RA, Brunetti A, Hill JM, Alger JR, Miletich RS, Francavilla TL, Di Chiro G. Role of iron and ferritin in MR imaging of the brain: a study in primates at different field strengths. Radiology. 1990;177:59–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

