Structure and function of the melanocortin2 receptor accessory protein (MRAP)
- PMID: 19028547
- PMCID: PMC2677758
- DOI: 10.1016/j.mce.2008.10.041
Structure and function of the melanocortin2 receptor accessory protein (MRAP)
Abstract
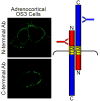
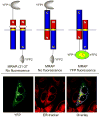
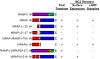
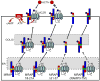
The melanocortin2 (MC2), or ACTH receptor, requires MC2 receptor accessory protein (MRAP) for function, and individuals lacking MRAP are ACTH-resistant and glucocorticoid-deficient. MRAP facilitates trafficking of the MC2 receptor to the plasma membrane and is absolutely required for ACTH binding and stimulation of cAMP. MRAP, which contains a single transmembrane domain, has a unique structure, an antiparallel homodimer. It can be isolated from the plasma membrane in a complex with the MC2 receptor. A short sequence just aminoterminal to the transmembrane domain of MRAP is essential for dual topology, while the transmembrane region is not; both are necessary for function. Deletion or alanine-substitution of other aminoterminal regions yields MRAP mutants that promote surface expression of the MC2 receptor but not receptor signaling. These results identify two distinct actions of MRAP: to permit trafficking of the MC2 receptor, and to allow surface receptor binding and signaling.
Figures

References
-
- Behrens M, Bartelt J, Reichling C, Winnig M, Kuhn C, Meyerhof W. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J Biol Chem. 2006;281:20650–20659. - PubMed
-
- Beltzer JP, Fiedler K, Fuhrer C, Geffen I, Handschin C, Wessels HP, Spiess M. Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J Biol Chem. 1991;266:973–978. - PubMed
-
- Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3:492–498. - PubMed
-
- Bernier V, Bichet DG, Bouvier M. Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol. 2004;4:528–533. - PubMed
-
- Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Gruters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

