Glucose- and glucokinase-controlled mal gene expression in Escherichia coli
- PMID: 19028900
- PMCID: PMC2632087
- DOI: 10.1128/JB.00767-08
Glucose- and glucokinase-controlled mal gene expression in Escherichia coli
Abstract
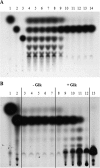
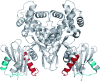
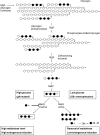
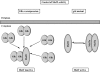
MalT is the central transcriptional activator of all mal genes in Escherichia coli. Its activity is controlled by the inducer maltotriose. It can be inhibited by the interaction with certain proteins, and its expression can be controlled. We report here a novel aspect of mal gene regulation: the effect of cytoplasmic glucose and glucokinase (Glk) on the activity and the expression of MalT. Amylomaltase (MalQ) is essential for the metabolism of maltose. It forms maltodextrins and glucose from maltose or maltodextrins. We found that glucose above a concentration of 0.1 mM blocked the activity of the enzyme. malQ mutants when grown in the absence of maltodextrins are endogenously induced by maltotriose that is derived from the degradation of glycogen. Therefore, the fact that glk malQ(+) mutants showed elevated mal gene expression finds its explanation in the reduced ability to remove glucose from MalQ-catalyzed maltodextrin formation and is caused by a metabolically induced MalQ(-) phenotype. However, even in mutants lacking glycogen, Glk controls endogenous induction. We found that overexpressed Glk due to its structural similarity with Mlc, the repressor of malT, binds to the glucose transporter (PtsG), releasing Mlc and thus increasing malT repression. In addition, even in mutants lacking Mlc (and glycogen), the overexpression of glk leads to a reduction in mal gene expression. We interpret this repression by a direct interaction of Glk with MalT concomitant with MalT inhibition. This repression was dependent on the presence of either maltodextrin phosphorylase or amylomaltase and led to the inactivation of MalT.
Figures



Similar articles
-
The maltodextrin system of Escherichia coli: metabolism and transport.J Bacteriol. 2005 Dec;187(24):8322-31. doi: 10.1128/JB.187.24.8322-8331.2005. J Bacteriol. 2005. PMID: 16321936 Free PMC article.
-
Network regulation of the Escherichia coli maltose system.J Mol Microbiol Biotechnol. 2002 May;4(3):301-7. J Mol Microbiol Biotechnol. 2002. PMID: 11931562 Review.
-
Molecular characterization of glucokinase from Escherichia coli K-12.J Bacteriol. 1997 Feb;179(4):1298-306. doi: 10.1128/jb.179.4.1298-1306.1997. J Bacteriol. 1997. PMID: 9023215 Free PMC article.
-
The maltodextrin system of Escherichia coli: glycogen-derived endogenous induction and osmoregulation.J Bacteriol. 2005 Dec;187(24):8332-9. doi: 10.1128/JB.187.24.8332-8339.2005. J Bacteriol. 2005. PMID: 16321937 Free PMC article.
-
Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation.Microbiol Mol Biol Rev. 1998 Mar;62(1):204-29. doi: 10.1128/MMBR.62.1.204-229.1998. Microbiol Mol Biol Rev. 1998. PMID: 9529892 Free PMC article. Review.
Cited by
-
The interplay between effector binding and allostery in an engineered protein switch.Protein Sci. 2016 Sep;25(9):1605-16. doi: 10.1002/pro.2962. Epub 2016 Jun 24. Protein Sci. 2016. PMID: 27272021 Free PMC article.
-
A comprehensive review on microbial production of 1,2-propanediol: micro-organisms, metabolic pathways, and metabolic engineering.Biotechnol Biofuels. 2021 Nov 18;14(1):216. doi: 10.1186/s13068-021-02067-w. Biotechnol Biofuels. 2021. PMID: 34794503 Free PMC article. Review.
-
A GTP-driven central carbon metabolism in the cellulolytic bacterium Ruminiclostridium cellulolyticum.Commun Biol. 2025 Mar 30;8(1):523. doi: 10.1038/s42003-025-07971-7. Commun Biol. 2025. PMID: 40159537 Free PMC article.
-
Transcriptome Analysis of Escherichia coli during dGTP Starvation.J Bacteriol. 2016 May 13;198(11):1631-44. doi: 10.1128/JB.00218-16. Print 2016 Jun 1. J Bacteriol. 2016. PMID: 27002130 Free PMC article.
-
Genome-scale metabolic network reconstruction and in silico flux analysis of the thermophilic bacterium Thermus thermophilus HB27.Microb Cell Fact. 2014 Apr 28;13:61. doi: 10.1186/1475-2859-13-61. Microb Cell Fact. 2014. PMID: 24774833 Free PMC article.
References
-
- Böhm, A., and W. Boos. 2004. Gene regulation in prokaryotes by subcellular relocalization of transcription factors. Curr. Opin. Microbiol. 7151-156. - PubMed
-
- Böhm, A., and W. Boos. 2004. Transport-dependent gene regulation by sequestration of transcriptional regulators, p. 47-66. In E. Boles and R. Krämer (ed.), Molecular mechanisms controlling transmembrane transport. Springer, Heidelberg, Germany.
-
- Boos, W., and A. Böhm. 2000. Learning new tricks from an old dog: MaIT of the Escherichia coli maltose system is part of a complex regulatory network. Trends Genet. 16404-409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

