Type 5 phosphodiesterase expression is a critical determinant of the endothelial cell angiogenic phenotype
- PMID: 19028977
- PMCID: PMC2643995
- DOI: 10.1152/ajplung.90474.2008
Type 5 phosphodiesterase expression is a critical determinant of the endothelial cell angiogenic phenotype
Abstract
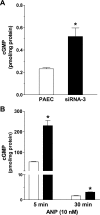
Type 5 phosphodiesterase (PDE5) inhibitors increase endothelial cell cGMP and promote angiogenesis. However, not all endothelial cell phenotypes express PDE5. Indeed, whereas conduit endothelial cells express PDE5, microvascular endothelial cells do not express this enzyme, and they are rapidly angiogenic. These findings bring into question whether PDE5 activity is a critical determinant of the endothelial cell angiogenic potential. To address this question, human full-length PDE5A1 was stably expressed in pulmonary microvascular endothelial cells. hPDE5A1 expression reduced the basal and atrial natriuretic peptide (ANP)-stimulated cGMP concentrations in these cells. hPDE5A1-expressing cells displayed attenuated network formation on Matrigel in vitro and also produced fewer blood vessels in Matrigel plug assays in vivo; the inhibitory actions of hPDE5A1 were reversed using sildenafil. To examine whether endogenous PDE5 activity suppresses endothelial cell angiogenic potential, small interfering RNA (siRNA) constructs were stably expressed in pulmonary artery endothelial cells. siRNA selectively decreased PDE5 expression and increased basal and ANP-stimulated cGMP concentrations in these conduit cells. PDE5 downregulation increased network formation on Matrigel in vitro and increased blood vessel formation in Matrigel plug assays in vivo. Collectively, our results indicate that PDE5 activity is an essential determinant of angiogenesis and suggest that PDE5 downregulation in microvascular endothelium imparts a stable, enhanced angiogenic potential to this cell type.
Figures






References
-
- Alvarez DF, Huang L, King JA, Elzarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008. - PubMed
-
- Benedict N, Seybert A, Mathier MA. Evidence-based pharmacologic management of pulmonary arterial hypertension. Clin Ther 29: 2134–2153, 2007. - PubMed
-
- Carmeliet P Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

