Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity
- PMID: 19029059
- PMCID: PMC2721473
- DOI: 10.1177/1073858408322675
Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity
Abstract
Many aspects of synaptic development, plasticity, and neurotransmission are critically influenced by NMDA-type glutamate receptors (NMDARs). Moreover, dysfunction of NMDARs has been implicated in a broad array of neurological disorders, including schizophrenia, stroke, epilepsy, and neuropathic pain. Classically, NMDARs were thought to be exclusively postsynaptic. However, substantial evidence in the past 10 years demonstrates that NMDARs also exist presynaptically and that presynaptic NMDA receptors (preNMDARs) modulate synapse function and have critical roles in plasticity at many synapses. Here the authors review current knowledge of the role of preNMDARs in synaptic transmission and plasticity, focusing on the neocortex. They discuss the prevalence, function, and development of these receptors, and their potential modification by experience and in brain pathology.
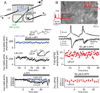
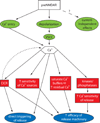
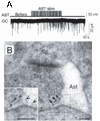
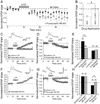
Figures





References
-
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. - PubMed
-
- Avanzini G, Franceschetti S. Cellular biology of epileptogenesis. Lancet Neurol. 2003;2:33–42. - PubMed
-
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

