The synergistic inhibition of atherogenesis in apoE-/- mice between pravastatin and the sPLA2 inhibitor varespladib (A-002)
- PMID: 19029066
- PMCID: PMC2656655
- DOI: 10.1194/jlr.M800361-JLR200
The synergistic inhibition of atherogenesis in apoE-/- mice between pravastatin and the sPLA2 inhibitor varespladib (A-002)
Abstract
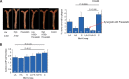
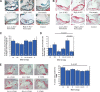
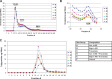
Secretory phospholipase A2 (sPLA2) activity promotes foam cell formation, increases proinflammatory bioactive lipid levels, decreases HDL levels, increases atherosclerosis in transgenic mice, and is an independent marker of cardiovascular disease. The effects of the sPLA2 inhibitor A-002 (varespladib) and pravastatin as monotherapies and in combination on atherosclerosis, lipids, and paraoxonase (PON) activity in apoE(-/-) mice were investigated. Male apoE(-/-) mice were placed on a 12-week high-fat diet supplemented with A-002 alone or combined with pravastatin. Atherosclerotic lesions were examined for size and composition using en face analysis, Movat staining, anti-CD68, and anti-alpha actin antibodies. Plasma lipids and PON activity were measured. A-002 decreased atherosclerotic lesion area by approximately 75% while increasing fibrous cap size by over 200%. HDL levels increased 40% and plasma PON activity increased 80%. Pravastatin monotherapy had no effect on lesion size but when combined with A-002, decreased lesion area 50% and total cholesterol levels 18% more than A-002 alone. A-002, a sPLA2 inhibitor, acts synergistically with pravastatin to decrease atherosclerosis, possibly through decreased levels of systemic inflammation or decreased lipid levels. A-002 treatment also resulted in a profound increase in plasma PON activity and significantly larger fibrous caps, suggesting the formation of more stable plaque architecture.
Figures
References
-
- Dennis E. A. 1994. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 269 13057–13060. - PubMed
-
- Wooton-Kee C. R., B. B. Boyanovsky, M. S. Nasser, W. J. de Villiers, and N. R. Webb. 2004. Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler. Thromb. Vasc. Biol. 24 762–767. - PubMed
-
- Leitinger N., A. D. Watson, S. Y. Hama, B. Ivandic, J. H. Qiao, J. Huber, K. F. Faull, D. S. Grass, M. Navab, A. M. Fogelman, et al. 1999. Role of group II secretory phospholipase A2 in atherosclerosis: 2. Potential involvement of biologically active oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 19 1291–1298. - PubMed
-
- Ivandic B., L. W. Castellani, X. P. Wang, J. H. Qiao, M. Mehrabian, M. Navab, A. M. Fogelman, D. S. Grass, M. E. Swanson, M. C. de Beer, et al. 1999. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler. Thromb. Vasc. Biol. 19 1284–1290. - PubMed
-
- Bostrom M. A., B. B. Boyanovsky, C. T. Jordan, M. P. Wadsworth, D. J. Taatjes, F. C. de Beer, and N. R. Webb. 2007. Group v secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler. Thromb. Vasc. Biol. 27 600–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

