Drug use in children: cohort study in three European countries
- PMID: 19029175
- PMCID: PMC2593449
- DOI: 10.1136/bmj.a2245
Drug use in children: cohort study in three European countries
Abstract
Objective: To provide an overview of drug use in children in three European countries.
Design: Retrospective cohort study, 2000-5.
Setting: Primary care research databases in the Netherlands (IPCI), United Kingdom (IMS-DA), and Italy (Pedianet).
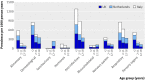
Participants: 675 868 children aged up to 14 (Italy) or 18 (UK and Netherlands).
Main outcome measure: Prevalence of use per year calculated by drug class (anatomical and therapeutic). Prevalence of "recurrent/chronic" use (three or more prescriptions a year) and "non-recurrent" or "acute" use (less than three prescriptions a year) within each therapeutic class. Descriptions of the top five most commonly used drugs evaluated for off label status within each anatomical class.
Results: Three levels of drug use could be distinguished in the study population: high (>10/100 children per year), moderate (1-10/100 children per year), and low (<1/100 children per year). For all age categories, anti-infective, dermatological, and respiratory drugs were in the high use group, whereas cardiovascular and antineoplastic drugs were always in the low use group. Emollients, topical steroids, and asthma drugs had the highest prevalence of recurrent use, but relative use of low prevalence drugs was more often recurrent than acute. In the top five highest prevalence drugs topical inhaled and systemic steroids, oral contraceptives, and topical or systemic antifungal drugs were most commonly used off label.
Conclusion: This overview of outpatient paediatric prescription patterns in a large European population could provide information to prioritise paediatric therapeutic research needs.
Conflict of interest statement
Competing interests: MCJMS has received various unconditional research grants from pharmaceutical companies (Merck, Pfizer, Johnson and Johnson, Amgen, Roche, Altana, GSK) and is consultant to Pfizer, Celgene, Servier, and Sanofi Aventis. AN has been reimbursed by Pfizer for attending several conferences. GP has received unconditional research grants from Merck and BMS). CG has received fees for speaking, consulting, and research from Sanofi Pasteur, GSK, Abbott, BMS, Gilead, Abbott, Tibotec, Boheringer Ingelheim, GSK-Biologicals). LC has received research grants from GSK, Abbott, Merck, and BMS.
Figures
References
-
- Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr 2005;164:552-8. - PubMed
-
- Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use. Paediatric regulation. Official Journal of the European Union 2006;18:L378/1.
-
- US Food and Drug Administration. Pediatric exclusivity labeling changes as of January 5, 2005. Rockville, MD: 2005.
-
- Davies A, Bateman M, Yates A, Bruno M. Pediatric regulations in Europe & the US. Regulatory Affairs Focus 2005;10:18-22.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical