Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat
- PMID: 19029185
- PMCID: PMC2670055
- DOI: 10.1113/jphysiol.2008.163162
Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat
Abstract
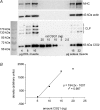
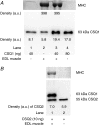
Whilst calsequestrin (CSQ) is widely recognized as the primary Ca2+ buffer in the sarcoplasmic reticulum (SR) in skeletal muscle fibres, its total buffering capacity and importance have come into question. This study quantified the absolute amount of CSQ isoform 1 (CSQ1, the primary isoform) present in rat extensor digitorum longus (EDL) and soleus fibres, and related this to their endogenous and maximal SR Ca2+ content. Using Western blotting, the entire constituents of minute samples of muscle homogenates or segments of individual muscle fibres were compared with known amounts of purified CSQ1. The fidelity of the analysis was proven by examining the relative signal intensity when mixing muscle samples and purified CSQ1. The CSQ1 contents of EDL fibres, almost exclusively type II fibres, and soleus type I fibres [SOL (I)] were, respectively, 36 +/- 2 and 10 +/- 1 micromol (l fibre volume)(-1), quantitatively accounting for the maximal SR Ca2+ content of each. Soleus type II [SOL (II)] fibres (approximately 20% of soleus fibres) had an intermediate amount of CSQ1. Every SOL (I) fibre examined also contained some CSQ isoform 2 (CSQ2), which was absent in every EDL and other type II fibre except for trace amounts in one case. Every EDL and other type II fibre had a high density of SERCA1, the fast-twitch muscle sarco(endo)plasmic reticulum Ca2+-ATPase isoform, whereas there was virtually no SERCA1 in any SOL (I) fibre. Maximal SR Ca2+ content measured in skinned fibres increased with CSQ1 content, and the ratio of endogenous to maximal Ca2+ content was inversely correlated with CSQ1 content. The relative SR Ca2+ content that could be maintained in resting cytoplasmic conditions was found to be much lower in EDL fibres than in SOL (I) fibres (approximately 20 versus >60%). Leakage of Ca2+ from the SR in EDL fibres could be substantially reduced with a SR Ca2+ pump blocker and increased by adding creatine to buffer cytoplasmic [ADP] at a higher level, both results indicating that at least part of the Ca2+ leakage occurred through SERCA. It is concluded that CSQ1 plays an important role in EDL muscle fibres by providing a large total pool of releasable Ca2+ in the SR whilst maintaining free [Ca2+] in the SR at sufficiently low levels that Ca2+ leakage through the high density of SERCA1 pumps does not metabolically compromise muscle function.
Figures



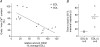
References
-
- Ahern GP, Junankar PR, Dulhunty AF. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994;352:369–374. - PubMed
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. - PubMed
-
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. J Muscle Res Cell Motil. 1996;17:55–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

