Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection
- PMID: 19029299
- PMCID: PMC2632039
- DOI: 10.1128/IAI.01050-08
Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection
Abstract
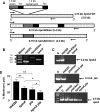
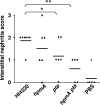
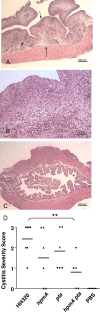
Complicated urinary tract infections (UTI) caused by Proteus mirabilis are associated with severe pathology in the bladder and kidney. To investigate the roles of two established cytotoxins, the HpmA hemolysin, a secreted cytotoxin, and proteus toxic agglutinin (Pta), a surface-associated cytotoxin, mutant analysis was used in conjunction with a mouse model of ascending UTI. Inactivation of pta, but not inactivation of hpmA, resulted in significant decreases in the bacterial loads of the mutant in kidneys (P < 0.01) and spleens (P < 0.05) compared to the bacterial loads of the wild type; the 50% infective dose (ID(50)) of an isogenic pta mutant or hpmA pta double mutant was 100-fold higher (5 x 10(8) CFU) than the ID(50) of parent strain HI4320 (5 x 10(6) CFU). Colonization by the parent strain caused severe cystitis and interstitial nephritis as determined by histopathological examination. Mice infected with the same bacterial load of the hpmA pta double mutant showed significantly reduced pathology (P < 0.01), suggesting that the additive effect of these two cytotoxins is critical during Proteus infection. Since Pta is surface associated and important for the persistence of P. mirabilis in the host, it was selected as a vaccine candidate. Mice intranasally vaccinated with a site-directed (indicated by an asterisk) (S366A) mutant purified intact toxin (Pta*) or the passenger domain Pta-alpha*, each independently conjugated with cholera toxin (CT), had significantly lower bacterial counts in their kidneys ( P = 0.001) and spleens (P = 0.002) than mice that received CT alone. The serum immunoglobulin G levels correlated with protection (P = 0.03). This is the first report describing the in vivo cytotoxicity and antigenicity of an autotransporter in P. mirabilis and its use in vaccine development.
Figures





References
-
- Alamuri, P., and H. L. Mobley. 2008. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 68997-1017. - PubMed
-
- Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 61583-1591. - PubMed
-
- Braun, V., and T. Focareta. 1991. Pore-forming bacterial protein hemolysins (cytolysins). Crit. Rev. Microbiol. 18115-158. - PubMed
-
- Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3320-332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

