Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis
- PMID: 19029300
- PMCID: PMC2632031
- DOI: 10.1128/IAI.00873-08
Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis
Abstract
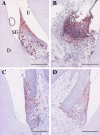
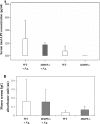
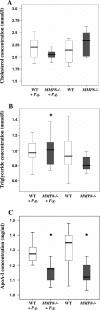
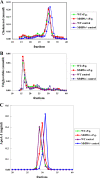
Periodontitis is a bacterium-induced chronic inflammation that destroys tissues that attach teeth to jaw bone. Pathologically excessive matrix metalloproteinase 8 (MMP-8) is among the key players in periodontal destruction by initiating type I collagen degradation. We studied MMP-8 in Porphyromonas gingivalis-induced periodontitis by using MMP-8-deficient (MMP8(-/-)) and wild-type (WT) mice. Alveolar bone loss, inflammatory mediator expression, serum immunoglobulin, and lipoprotein responses were investigated to clarify the role of MMP-8 in periodontitis and systemic inflammatory responses. P. gingivalis infection induced accelerated site-specific alveolar bone loss in both MMP8(-/-) and WT mice relative to uninfected mice. The most extensive bone degradation took place in the P. gingivalis-infected MMP8(-/-) group. Surprisingly, MMP-8 significantly attenuated (P < 0.05) P. gingivalis-induced site-specific alveolar bone loss. Increased alveolar bone loss in P. gingivalis-infected MMP8(-/-) and WT mice was associated with increase in gingival neutrophil elastase production. Serum lipoprotein analysis demonstrated changes in the distribution of high-density lipoprotein (HDL) and very-low-density lipoprotein (VLDL) particles; unlike the WT mice, the MMP8(-/-) mice underwent a shift toward a smaller HDL/VLDL particle sizes. P. gingivalis infection increased the HDL/VLDL particle size in the MMP8(-/-) mice, which is an indicator of lipoprotein responses during systemic inflammation. Serum total lipopolysaccharide activity and the immunoglobulin G-class antibody level in response to P. gingivalis were significantly elevated in both infected mice groups. Thus, MMP-8 appears to act in a protective manner inhibiting the development of bacterium-induced periodontal tissue destruction, possibly through the processing anti-inflammatory cytokines and chemokines. Bacterium-induced periodontitis, especially in MMP8(-/-) mice, is associated with systemic inflammatory and lipoprotein changes that are likely involved in early atherosclerosis.
Figures


References
-
- Assuma, R., T. Oates, D. Cochran, S. Amar, and D. T. Graves. 1998. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 160403-409. - PubMed
-
- Balbín, M., A. Fueyo, A. M. Tester, A. M. Pendás, A. S. Pitiot, A. Astudillo, C. M. Overall, S. D. Shapiro, and C. Lopéz-Otín. 2003. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 35252-257. - PubMed
-
- Barlage, S., D. Fröhlich, A. Böttcher, M. Jauhiainen, H. P. Müller, F. Noetzel, G. Rothe, C. Schütt, R. P. Linke, K. J. Lackner, C. Ehnholm, and G. Scmitz. 2001. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J. Lipid Res. 42281-290. - PubMed
-
- Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 671123-1137. - PubMed
-
- Bezerra, M. M., V. de Lima, V. B. M. Alencar, I. B. Vieira, G. A. C. Brito, R. A. Ribeiro, and F. A. C. Rocha. 2000. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 711009-1014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

