Deadenylation is a widespread effect of miRNA regulation
- PMID: 19029310
- PMCID: PMC2612776
- DOI: 10.1261/rna.1399509
Deadenylation is a widespread effect of miRNA regulation
Abstract
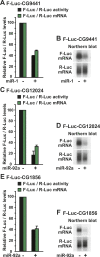
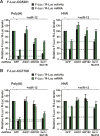
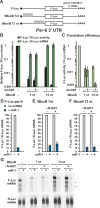
miRNAs silence gene expression by repressing translation and/or by promoting mRNA decay. In animal cells, degradation of partially complementary miRNA targets occurs via deadenylation by the CAF1-CCR4-NOT1 deadenylase complex, followed by decapping and subsequent exonucleolytic digestion. To determine how generally miRNAs trigger deadenylation, we compared mRNA expression profiles in D. melanogaster cells depleted of AGO1, CAF1, or NOT1. We show that approximately 60% of AGO1 targets are regulated by CAF1 and/or NOT1, indicating that deadenylation is a widespread effect of miRNA regulation. However, neither a poly(A) tail nor mRNA circularization are required for silencing, because mRNAs whose 3' ends are generated by a self-cleaving ribozyme are also silenced in vivo. We show further that miRNAs trigger mRNA degradation, even when binding by 40S ribosomal subunits is inhibited in cis. These results indicate that miRNAs promote mRNA decay by altering mRNP composition and/or conformation, rather than by directly interfering with the binding and function of ribosomal subunits.
Figures





References
-
- Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
