Slow inactivation in Shaker K channels is delayed by intracellular tetraethylammonium
- PMID: 19029372
- PMCID: PMC2585862
- DOI: 10.1085/jgp.200810057
Slow inactivation in Shaker K channels is delayed by intracellular tetraethylammonium
Abstract
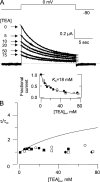
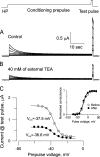
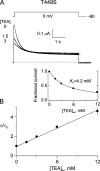
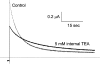
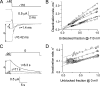
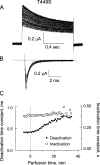
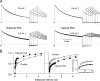
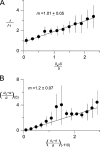
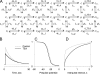
After removal of the fast N-type inactivation gate, voltage-sensitive Shaker (Shaker IR) K channels are still able to inactivate, albeit slowly, upon sustained depolarization. The classical mechanism proposed for the slow inactivation observed in cell-free membrane patches--the so called C inactivation--is a constriction of the external mouth of the channel pore that prevents K(+) ion conduction. This constriction is antagonized by the external application of the pore blocker tetraethylammonium (TEA). In contrast to C inactivation, here we show that, when recorded in whole Xenopus oocytes, slow inactivation kinetics in Shaker IR K channels is poorly dependent on external TEA but severely delayed by internal TEA. Based on the antagonism with internally or externally added TEA, we used a two-pulse protocol to show that half of the channels inactivate by way of a gate sensitive to internal TEA. Such gate had a recovery time course in the tens of milliseconds range when the interpulse voltage was -90 mV, whereas C-inactivated channels took several seconds to recover. Internal TEA also reduced gating charge conversion associated to slow inactivation, suggesting that the closing of the internal TEA-sensitive inactivation gate could be associated with a significant amount of charge exchange of this type. We interpreted our data assuming that binding of internal TEA antagonized with U-type inactivation (Klemic, K.G., G.E. Kirsch, and S.W. Jones. 2001. Biophys. J. 81:814-826). Our results are consistent with a direct steric interference of internal TEA with an internally located slow inactivation gate as a "foot in the door" mechanism, implying a significant functional overlap between the gate of the internal TEA-sensitive slow inactivation and the primary activation gate. But, because U-type inactivation is reduced by channel opening, trapping the channel in the open conformation by TEA would also yield to an allosteric delay of slow inactivation. These results provide a framework to explain why constitutively C-inactivated channels exhibit gating charge conversion, and why mutations at the internal exit of the pore, such as those associated to episodic ataxia type I in hKv1.1, cause severe changes in inactivation kinetics.
Figures



References
-
- Ayer, R.K. Jr., and F.J. Sigworth. 1997. Enhanced closed-state inactivation in a mutant Shaker K+ channel. J. Membr. Biol. 157:215–230. - PubMed
-
- Basso, C., P. Labarca, E. Stefani, O. Alvarez, and R. Latorre. 1998. Pore accessibility during C-type inactivation in Shaker K+ channels. FEBS Lett. 429:375–380. - PubMed
-
- Bernèche, S., and B. Roux. 2005. A gate in the selectivity filter of potassium channels. Structure. 13:591–600. - PubMed
-
- Bezanilla, F. 2000. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80:555–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

