Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma
- PMID: 19029405
- PMCID: PMC2664986
- DOI: 10.1369/jhc.2008.952044
Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma
Abstract
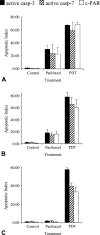
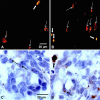
Immunohistochemistry to active caspase-3, recently recommended for apoptosis detection, is inappropriate to detect apoptosis involving caspase-7. Cleavage of poly-ADP-ribose polymerase 1 (PARP-1), a major substrate of both caspases, is a valuable marker of apoptosis. Apoptosis evaluation induced in vitro either by paclitaxel or by photodynamic treatment (PDT) with Foscan in HT29 or KB monolayer cells and HT29 spheroids yielded a close percentage of labeled cells whatever the antibody used, whereas in control specimens, cleaved PARP (c-PARP) immunostaining failed to detect apoptosis as efficiently as active caspase-3 or -7 immunostaining. Studies in MDA-MB231 monolayer cells and HT29 xenografts either subjected or not subjected to Foscan-PDT resulted in a significant higher number of active caspase-3-labeled cells, although immunofluorescence analysis showed c-PARP and active caspase-3 perfectly colocalized in tumors. A restricted expression of c-PARP was obvious in the greater part of caspase-3 expressing cells from control tumor, whereas photosensitized tumors showed a higher number of cells expressing large fluorescent spots from both active caspase-3 and c-PARP. These results support the assumption that c-PARP expression was dependent on treatment-induced apoptosis. The absence of caspase-7 activation in some caspase-3-expressing cells undergoing Foscan-PDT shows the relevance of using antibodies that can discriminate caspase-dependent apoptotic pathways.
Figures



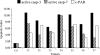

References
-
- Alvarez-Gonzalez R, Spring H, Muller M, Burkle A (1999) Selective loss of poly(ADP-ribose) and the 85-kDa fragment of poly(ADP-ribose) polymerase in nucleoli during alkylation-induced apoptosis of HeLa cells. J Biol Chem 274:32122–32126 - PubMed
-
- Coutier S, Bezdetnaya LN, Foster TH, Parache RM, Guillemin F (2002) Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in meta-tetra (hydroxyphenyl) chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat Res 158:339–345 - PubMed
-
- D'Amours D, Sallmann FR, Dixit VM, Poirier GG (2001) Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci 114:3771–3778 - PubMed
-
- Davidson DJ, Haskell C, Majest S, Kherzai A, Egan DA, Walter KA, Schneider A, et al. (2005) Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface-expressed glucose-regulated protein 78. Cancer Res 65:4663–4672 - PubMed
-
- Decker P, Muller S (2002) Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol 3:275–283 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

