Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer
- PMID: 19029421
- PMCID: PMC3864402
- DOI: 10.1200/JCO.2007.15.9830
Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer
Abstract
Purpose: We conducted a phase III trial in patients with previously untreated metastatic prostate cancer to test the hypothesis that three 8-week cycles of ketoconazole and doxorubicin alternating with vinblastine and estramustine, given in addition to standard androgen deprivation, would delay the appearance of castrate-resistant disease.
Patients and methods: Eligible patients had metastatic prostate cancer threatening enough to justify sustained androgen ablation and were fit enough for chemotherapy. The primary end point was time to castrate-resistant progression as shown by increasing prostate-specific antigen, new radiographic lesions, worsening cancer-related symptoms, or receipt of any other systemic therapy.
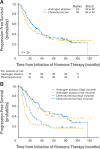
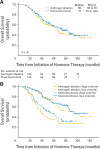
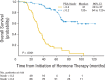
Results: Three hundred six patients were registered; 286 are reported. Median time to progression was 24 months (95% CI, 18 to 39 months) in the standard therapy arm, and 35 months (95% CI, 26 to 44 months) in the chemohormonal group (P = .39). At median follow-up of 6.4 years, overall survival was 5.4 years (95% CI, 4.7 to 7.8 years) in the standard therapy arm versus 6.1 years (95% CI, 5.1 to 10.1 years; P = .41). Prostate-specific antigen kinetics at the time of androgen ablation and the nadir after hormone treatment were strongly correlated with survival. Chemotherapy significantly increased the burden of therapy, with 51% of patients experiencing an adverse event of grade 3 or worse, especially thromboembolic events.
Conclusion: There is no role for ketoconazole and doxorubicin alternating with vinblastine and estramustine before emergence of a castrate-resistant phenotype.
Figures

References
-
- Jemal A, Siegel R, Ward E, et al: Cancer Statistics, 2007. CA Cancer J Clin 57:43-66, 2007 - PubMed
-
- Yagoda A, Petrylak D: Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer 71:1098-1109, 1993 - PubMed
-
- Beer T, Raghavan D: Chemotherapy for hormone-refractory prostate cancer: Beauty is in the eye of the beholder. Prostate 45:184-193, 2000 - PubMed
-
- Ellerhorst JA, Tu SM, Amato RJ, et al: Phase II trial of alternating weekly chemohormonal therapy for patients with androgen-independent prostate cancer. Clin Cancer Res 3:2371-2376, 1997 - PubMed
-
- Smith DC, Esper P, Strawderman M, et al: Phase II trial of oral estramustine, oral etoposide, and intravenous paclitaxel in hormone-refractory prostate cancer. J Clin Oncol 17:1664-1671, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

