Subcutaneous IGF-1 is not beneficial in 2-year ALS trial
- PMID: 19029516
- PMCID: PMC2617770
- DOI: 10.1212/01.wnl.0000335970.78664.36
Subcutaneous IGF-1 is not beneficial in 2-year ALS trial
Abstract
Background: Previous human clinical trials of insulin-like growth factor type I (IGF-1) in amyotrophic lateral sclerosis (ALS) have been inconsistent. This phase III, randomized, double-blind, placebo-controlled study was undertaken to address whether IGF-1 benefited patients with ALS.
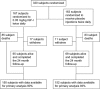
Methods: A total of 330 patients from 20 medical centers were randomized to receive 0.05 mg/kg body weight of human recombinant IGF-1 given subcutaneously twice daily or placebo for 2 years. The primary outcome measure was change in their manual muscle testing score. Secondary outcome measures included tracheostomy-free survival and rate of change in the revised ALS functional rating scale. Intention to treat analysis was used.
Results: There was no difference between treatment groups in the primary or secondary outcome measures after the 2-year treatment period.
Conclusions: Insulin-like growth factor type I does not provide benefit for patients with amyotrophic lateral sclerosis.
Figures
Comment in
-
Subcutaneous IGF-1 is not beneficial in 2-year ALS trial.Neurology. 2009 Oct 13;73(15):1247; author reply 1247-8. doi: 10.1212/WNL.0b013e3181b26ae6. Neurology. 2009. PMID: 19822878 No abstract available.
References
-
- Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis 1999;6:335–346. - PubMed
-
- Vergani L, Losa M, Lesma E, et al. Glycosaminoglycans boost insulin-like growth factor-I-promoted neuroprotection: blockade of motor neuron death in the wobbler mouse. Neuroscience 1999;93:565–572. - PubMed
-
- Vergani L, Finco C, Di Giulio AM, Muller EE, Gorio A. Effects of low doses of glycosaminoglycans and insulin-like growth factor-I on motor neuron disease in wobbler mouse. Neurosci Lett 1997;228:41–44. - PubMed
-
- Borasio GD, Robberecht W, Leigh PN, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology 1998;51:583–586. - PubMed
-
- Lai EC, Felice KJ, Festoff BW, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS: a placebo-controlled study. The North America ALS/IGF-I Study Group.Neurology 1997;49:1621–1630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous