Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload
- PMID: 19030868
- PMCID: PMC2782393
- DOI: 10.1007/s00418-008-0541-5
Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload
Abstract
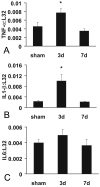
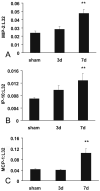
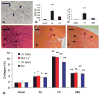
Myocardial fibrosis is an integral component of most cardiac pathologic conditions and contributes to the development of both systolic and diastolic dysfunction. Because of the availability of genetically manipulated animals, mouse models are essential for understanding the mechanisms involved in the pathogenesis of cardiac fibrosis. Accordingly, we characterized the inflammatory and fibrotic response in a mouse model of cardiac pressure overload due to transverse aortic constriction (TAC). Following TAC, mouse hearts exhibited induction of chemokines and proinflammatory cytokines, associated with macrophage, but not neutrophil, infiltration. Induction of inflammatory cytokines was followed by a late upregulation of transforming growth factor (TGF)-beta isoforms, activation of the Smad2/3 and Smad1/5 pathways, induction of matricellular proteins, and deposition of collagen. Inflammatory activity decreased after 28 days of TAC; at this timepoint established fibrosis was noted, accompanied by ventricular dilation and systolic dysfunction. Late induction of inhibitory mediators, such as TGF-beta, may play an essential role in the transition from inflammation to fibrosis by suppressing inflammatory gene synthesis while inducing matrix deposition. Our findings identify molecular mediators and pathways with a potential role in cardiac fibrosis laying the foundations for studies exploring the pathogenesis of fibrotic cardiac remodeling using genetically targeted mice.
Figures


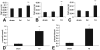


References
-
- Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. - PubMed
-
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. - PubMed
-
- Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

