Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis
- PMID: 19030911
- PMCID: PMC2693320
- DOI: 10.1007/s00395-008-0764-6
Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis
Abstract
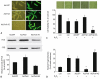
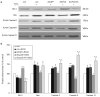
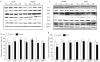
Peroxiredoxin II, a cytosolic isoform of the antioxidant enzyme family, has been implicated in cancer-associated cell death and apoptosis, but its functional role in the heart remains to be elucidated. Interestingly, the expression levels of peroxiredoxin II were decreased in mouse hearts upon ischemia-reperfusion, while they were elevated in two genetically modified hyperdynamic hearts with phospholamban ablation or protein phosphatase 1 inhibitor 1 overexpression. To delineate the functional significance of altered peroxiredoxin II expression, adenoviruses encoding sense or antisense peroxiredoxin II were generated; cardiomyocytes were infected, and then subjected to H(2)O(2) treatment to mimic oxidative stress-induced cell death and apoptosis. H(2)O(2) stimulation resulted in a significant decrease of endogenous peroxiredoxin II expression, along with reduced cell viability in control cells. However, overexpression of peroxiredoxin II significantly protected from H(2)O(2)-induced apoptosis and necrosis, while downregulation of this enzyme promoted the detrimental effects of oxidative stress in cardiomyocytes. The beneficial effects of peroxiredoxin II were associated with increased Bcl-2 expression, decreased expression of Bax and attenuated activity of caspases 3, 9 and 12. Furthermore, there were no significant alterations in the expression levels of the other five isoforms of peroxiredoxin, as well as active catalase or glutathione peroxidase-1 after ischemia-reperfusion or H(2)O(2) treatment. These findings suggest that peroxiredoxin II may be a unique antioxidant in the cardiac system and may represent a potential target for cardiac protection from oxidative stress-induced injury.
Figures



Similar articles
-
Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro.Acta Pharmacol Sin. 2013 Apr;34(4):487-95. doi: 10.1038/aps.2012.185. Epub 2013 Feb 11. Acta Pharmacol Sin. 2013. PMID: 23396376 Free PMC article.
-
AMPK Contributes to Cardioprotective Effects of Pterostilbene Against Myocardial Ischemia- Reperfusion Injury in Diabetic Rats by Suppressing Cardiac Oxidative Stress and Apoptosis.Cell Physiol Biochem. 2018;46(4):1381-1397. doi: 10.1159/000489154. Epub 2018 Apr 18. Cell Physiol Biochem. 2018. PMID: 29689567
-
Extracellular signal-regulated kinase 1/2 activation is involved in intermedin1-53 attenuating myocardial oxidative stress injury induced by ischemia/reperfusion.Peptides. 2012 Feb;33(2):329-35. doi: 10.1016/j.peptides.2011.12.016. Epub 2012 Jan 8. Peptides. 2012. PMID: 22244813
-
Effect of beta2-adrenergic agonist clenbuterol on ischemia/reperfusion injury in isolated rat hearts and cardiomyocyte apoptosis induced by hydrogen peroxide.Acta Pharmacol Sin. 2008 Jun;29(6):661-9. doi: 10.1111/j.1745-7254.2008.00794.x. Acta Pharmacol Sin. 2008. PMID: 18501112
-
N-acetylserotonin protects PC12 cells from hydrogen peroxide induced damage through ROS mediated PI3K / AKT pathway.Cell Cycle. 2022 Nov;21(21):2268-2282. doi: 10.1080/15384101.2022.2092817. Epub 2022 Jun 26. Cell Cycle. 2022. PMID: 35758219 Free PMC article. Review.
Cited by
-
Altered spatiotemporal dynamics of the mitochondrial membrane potential in the hypertrophied heart.Biophys J. 2010 May 19;98(10):2063-71. doi: 10.1016/j.bpj.2010.01.045. Biophys J. 2010. PMID: 20483313 Free PMC article.
-
Redox signaling in cardiac myocytes.Free Radic Biol Med. 2011 Apr 1;50(7):777-93. doi: 10.1016/j.freeradbiomed.2011.01.003. Epub 2011 Jan 12. Free Radic Biol Med. 2011. PMID: 21236334 Free PMC article. Review.
-
An investigation into the cytotoxic effects of 13-acetoxysarcocrassolide from the soft coral Sarcophyton crassocaule on bladder cancer cells.Mar Drugs. 2011 Dec;9(12):2622-2642. doi: 10.3390/md9122622. Epub 2011 Dec 13. Mar Drugs. 2011. PMID: 22363243 Free PMC article.
-
PRDX2 in Myocyte Hypertrophy and Survival is Mediated by TLR4 in Acute Infarcted Myocardium.Sci Rep. 2017 Aug 1;7(1):6970. doi: 10.1038/s41598-017-06718-7. Sci Rep. 2017. PMID: 28765537 Free PMC article.
-
SIRT1 is Required for Exercise-Induced Beneficial Effects on Myocardial Ischemia/Reperfusion Injury.J Inflamm Res. 2021 Apr 7;14:1283-1296. doi: 10.2147/JIR.S300997. eCollection 2021. J Inflamm Res. 2021. Retraction in: J Inflamm Res. 2022 Jul 20;15:4105-4106. doi: 10.2147/JIR.S382789. PMID: 33854356 Free PMC article. Retracted.
References
-
- Bao J, Sato K, Li M, Gao Y, Abid R, Aird W, Simons M, Post MJ. PR-39 and PR-11 peptides inhibit ischemia-reperfusion injury by blocking proteasome-mediated I kappa B alpha degradation. Am J Physiol Heart Circ Physiol. 2001;281:H2612–2618. - PubMed
-
- Campbell B, Adams J, Shin YK, Lefer AM. Cardioprotective effects of a novel proteasome inhibitor following ischemia and reperfusion in the isolated perfused rat heart. J Mol Cell Cardiol. 1999;31:467–476. - PubMed
-
- Casey TM, Arthur PG, Bogoyevitch MA. Necrotic death without mitochondrial dysfunction-delayed death of cardiac myocytes following oxidative stress. Biochim Biophys Acta. 2007;1773:342–351. - PubMed
-
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

