Probing the function of heme distortion in the H-NOX family
- PMID: 19032091
- PMCID: PMC2646007
- DOI: 10.1021/cb800185h
Probing the function of heme distortion in the H-NOX family
Abstract
Hemoproteins carry out diverse functions utilizing a wide range of chemical reactivity while employing the same heme prosthetic group. It is clear from high-resolution crystal structures and biochemical studies that protein-bound hemes are not planar and adopt diverse conformations. The crystal structure of an H-NOX domain from Thermoanaerobacter tengcongensis (Tt H-NOX) contains the most distorted heme reported to date. In this study, Tt H-NOX was engineered to adopt a flatter heme by mutating proline 115, a conserved residue in the H-NOX family, to alanine. Decreasing heme distortion in Tt H-NOX increases affinity for oxygen and decreases the reduction potential of the heme iron. Additionally, flattening the heme is associated with significant shifts in the N-terminus of the protein. These results show a clear link between the heme conformation and Tt H-NOX structure and demonstrate that heme distortion is an important determinant for maintaining biochemical properties in H-NOX proteins.
Figures

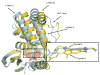

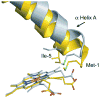
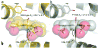
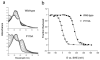
Comment in
-
A twist on heme signaling.ACS Chem Biol. 2008 Nov 21;3(11):673-5. doi: 10.1021/cb800269h. ACS Chem Biol. 2008. PMID: 19032089 Free PMC article.
References
-
- Shelnutt JA, Song XZ, Ma JG, Jia SL, Jentzen W, Medforth CJ. Nonplanar porphyrins and their significance in proteins. Chem Soc Rev. 1998;27:31–41.
-
- Zbylut SD, Kincaid JR. Resonance Raman evidence for protein-induced out-of-plane distortion of the heme prosthetic group of mammalian lactoperoxidase. J Amer Chem Soc. 2002;124:6751–6758. - PubMed
-
- Li D, Stuehr DJ, Yeh SR, Rousseau DL. Heme distortion modulated by ligand-protein interactions in inducible nitric oxide synthase. J Biol Chem. 2004;279:26489–26499. - PubMed
-
- Ravikanth M, Chandrashekar TK. Nonplanar porphyrins and their biological relevance - ground and excited-state dynamics. Coordination Chemistry. 1995;82:105–188.
-
- Weichsel A, Andersen JF, Roberts SA, Montfort WR. Nitric oxide binding to nitrophorin 4 induces complete distal pocket burial. Nature Structural Biology. 2000;7:551–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

