Flexible dosing of tincture of opium in the management of opioid withdrawal: pharmacokinetics and pharmacodynamics
- PMID: 19032172
- PMCID: PMC2661979
- DOI: 10.1111/j.1365-2125.2008.03277.x
Flexible dosing of tincture of opium in the management of opioid withdrawal: pharmacokinetics and pharmacodynamics
Abstract
Aims: The aim was to evaluate the clinical effectiveness, pharmacodynamics and pharmacokinetics of a range of Tincture of Opium (TOP) doses in the management of opioid withdrawal.
Methods: Forty-five opium-dependent Thai subjects were allocated to three dosing groups (6.66, 13.3 and 20 mg morphine equivalents, twice daily) depending on their self-reported prior opium use. On day 5 of dosing subjects underwent an interdosing interval study where blood, withdrawal scores, heart rate and blood pressure (BP) were collected at 0, 1, 3 and 8 h. Plasma morphine concentrations were quantified by high-performance liquid chromatography, and plasma morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) concentrations by LCMS.
Results: Thirty-two subjects completed the study. Withdrawal scores were low for all subjects (range 9-23% of maximum response). There were dose-dependent changes in both systolic and diastolic BP (P = 0.021 and P = 0.01, respectively), but these were not considered clinically significant. There were no effects of dose on respiratory rate. Plasma morphine concentrations changed significantly across the interdosing interval (P = 0.0001), rising to a maximum at 1 h after dosing. Plasma morphine concentrations also differed according to dose (P < 0.05). The mean ratios of the morphine glucuronides were found to be: M3G/M6G = 7.7, M3G/morphine = 35.6 and M6G/morphine = 4.9, values comparable to those previously reported.
Conclusion: The management of opioid withdrawal can be achieved, with minimal adverse effects, by using flexible dosing of TOP.
Figures
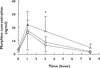
 ); Dose 2 (
); Dose 2 ( ); Dose 3 (
); Dose 3 ( )
)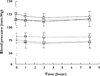
 ); SBP (Dose 2) (
); SBP (Dose 2) ( ); SBP (Dose 3) (
); SBP (Dose 3) ( ); DBP (Dose 1) (
); DBP (Dose 1) ( ); DBP (Dose 2) (
); DBP (Dose 2) ( ); DBP (Dose 3) (
); DBP (Dose 3) ( )
)
 ); Dose 2 (
); Dose 2 ( ); Dose 3 (
); Dose 3 ( )
)
 ); Dose 2 (
); Dose 2 ( ); Dose 3 (
); Dose 3 ( ) (B) Log withdrawal scores adjusted for prior daily opium use and plasma morphine concentration at each time point for all subjects. *P < 0.05; time point 1 h vs. 3 h
) (B) Log withdrawal scores adjusted for prior daily opium use and plasma morphine concentration at each time point for all subjects. *P < 0.05; time point 1 h vs. 3 hReferences
-
- Holmstrand J, Anggård E, Gunne LM. Methadone maintenance: plasma levels and therapeutic outcome. Clin Pharmacol Ther. 1978;23:175–80. - PubMed
-
- Schall U, Pries E, Katta T, Kloppel A, Gastpar M. Pharmacokinetic and pharmacodynamic interactions in an outpatient maintenance therapy of intravenous heroin users with levomethadone. Addict Biol. 1996;1:105–13. - PubMed
-
- Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. Lancet. 1999;353:221–6. - PubMed
-
- Bell J, Mattick R, Hay A, Chan J, Hall W. Methadone maintenance and drug-related crime. J Subst Abuse. 1997;9:15–25. - PubMed
-
- Grøndbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand. 1990;82:223–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

