Molecular mechanisms of alcoholic fatty liver
- PMID: 19032584
- PMCID: PMC2633431
- DOI: 10.1111/j.1530-0277.2008.00827.x
Molecular mechanisms of alcoholic fatty liver
Abstract
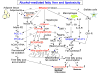
Alcoholic fatty liver is a potentially pathologic condition which can progress to steatohepatitis, fibrosis, and cirrhosis if alcohol consumption is continued. Alcohol exposure may induce fatty liver by increasing NADH/NAD(+) ratio, increasing sterol regulatory element-binding protein-1 (SREBP-1) activity, decreasing peroxisome proliferator-activated receptor-alpha (PPAR-alpha) activity, and increasing complement C3 hepatic levels. Alcohol may increase SREBP-1 activity by decreasing the activities of AMP-activated protein kinase and sirtuin-1. Tumor necrosis factor-alpha (TNF-alpha) produced in response to alcohol exposure may cause fatty liver by up-regulating SREBP-1 activity, whereas betaine and pioglitazone may attenuate fatty liver by down-regulating SREBP-1 activity. PPAR-alpha agonists have potentials to attenuate alcoholic fatty liver. Adiponectin and interleukin-6 may attenuate alcoholic fatty liver by up-regulating PPAR-alpha and insulin signaling pathways while down-regulating SREBP-1 activity and suppressing TNF-alpha production. Recent studies show that paracrine activation of hepatic cannabinoid receptor 1 by hepatic stellate cell-derived endocannabinoids also contributes to the development of alcoholic fatty liver. Furthermore, oxidative modifications and inactivation of the enzymes involved in the mitochondrial and/or peroxisomal beta-oxidation of fatty acids could contribute to fat accumulation in the liver.
Figures
References
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291(5513):2613–6. - PubMed
-
- Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;364(4):972–7. - PubMed
-
- Aleynik SI, Lieber CS. Polyenylphosphatidylcholine corrects the alcohol-induced hepatic oxidative stress by restoring S-adenosylmethionine. Alcohol Alcohol. 2003;38(3):208–12. - PubMed
-
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273(10):5678–84. - PubMed
-
- Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32(1):11–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

