Lipid bilayer composition influences small multidrug transporters
- PMID: 19032749
- PMCID: PMC2605743
- DOI: 10.1186/1471-2091-9-31
Lipid bilayer composition influences small multidrug transporters
Abstract
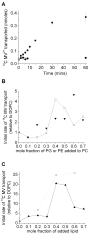
Background: Membrane proteins are influenced by their surrounding lipids. We investigate the effect of bilayer composition on the membrane transport activity of two members of the small multidrug resistance family; the Escherichia coli transporter, EmrE and the Mycobacterium tuberculosis, TBsmr. In particular we address the influence of phosphatidylethanolamine and anionic lipids on the activity of these multidrug transporters. Phosphatidylethanolamine lipids are native to the membranes of both transporters and also alter the lateral pressure profile of a lipid bilayer. Lipid bilayer lateral pressures affect membrane protein insertion, folding and activity and have been shown to influence reconstitution, topology and activity of membrane transport proteins.
Results: Both EmrE and TBsmr are found to exhibit a similar dependence on lipid composition, with phosphatidylethanolamine increasing methyl viologen transport. Anionic lipids also increase transport for both EmrE and TBsmr, with the proteins showing a preference for their most prevalent native anionic lipid headgroup; phosphatidylglycerol for EmrE and phosphatidylinositol for TBsmr.
Conclusion: These findings show that the physical state of the membrane modifies drug transport and that substrate translocation is dependent on in vitro lipid composition. Multidrug transport activity seems to respond to alterations in the lateral forces exerted upon the transport proteins by the bilayer.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

