Identification of possible candidate genes regulating Sjögren's syndrome-associated autoimmunity: a potential role for TNFSF4 in autoimmune exocrinopathy
- PMID: 19032782
- PMCID: PMC2656241
- DOI: 10.1186/ar2560
Identification of possible candidate genes regulating Sjögren's syndrome-associated autoimmunity: a potential role for TNFSF4 in autoimmune exocrinopathy
Abstract
Introduction: Sjögren syndrome (SjS) is a systemic autoimmune disease in which an immunological attack primarily against the salivary and lacrimal glands results in the loss of acinar cell tissue and function, leading to stomatitis sicca and keratoconjunctivitis sicca. In recent years, two genetic regions, one on chromosome 1 (designated autoimmune exocrinopathy 2 or Aec2) and the second on chromosome 3 (designated autoimmune exocrinopathy 1 or Aec1) derived from nonobese diabetic (NOD) mice, have been shown to be necessary and sufficient to replicate SjS-like disease in nonsusceptible C57BL/6 mice.
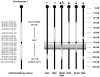
Methods: Starting with the SjS-susceptible C57BL/6-derived mouse, referred to as C57BL/6.NOD-Aec1Aec2, we generated a large set of recombinant inbred (RI) lines containing portions of Aec2 as a means of identifying more precisely the genetic elements of chromosome 1 responsible for disease development.
Results: Disease profiling of these RI lines has revealed that the SjS susceptibility genes of Aec2 lie within a region located at approximately 79 +/- 5 cM distal to the centromere, as defined by microsatellite markers. This chromosomal region contains several sets of genes known to correlate with various immunopathological features of SjS as well as disease susceptibility genes for both type 1 diabetes and systemic lupus erythematosus in mice. One gene in particular, tumor necrosis factor (ligand) superfamily member 4 (or Ox40 ligand), encoding a product whose biological functions correlate with both physiological homeostasis and immune regulations, could be a potential candidate SjS susceptibility gene.
Conclusions: These new RI lines represent the first step not only in fine mapping SjS susceptibility loci but also in identifying potential candidate SjS susceptibility genes. Identification of possible candidate genes permits construction of models describing underlying molecular pathogenic mechanisms in this model of SjS and establishes a basis for construction of specific gene knockout mice.
Figures

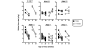



Comment in
-
Genetic control of disease in an experimental model for Sjögren's syndrome.Arthritis Res Ther. 2009;11(1):102. doi: 10.1186/ar2583. Epub 2009 Jan 20. Arthritis Res Ther. 2009. PMID: 19216731 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

