Lipid activation of protein kinases
- PMID: 19033211
- PMCID: PMC2674703
- DOI: 10.1194/jlr.R800064-JLR200
Lipid activation of protein kinases
Abstract
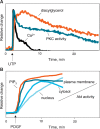
Lipids acutely control the amplitude, duration, and subcellular location of signaling by lipid second messenger-responsive kinases. Typically, this activation is controlled by membrane-targeting modules that allosterically control the function of kinase domains within the same polypeptide. Protein kinase C (PKC) has served as the archetypal lipid-regulated kinase, providing a prototype for lipid-controlled kinase activation that is followed by kinases throughout the kinome, including its close cousin, Akt (protein kinase B). This review addresses the molecular mechanisms by which PKC and Akt transduce signals propagated by the two major lipid second messenger pathways in cells, those of diacylglycerol signaling and phosphatidylinositol-3,4,5-trisphosphate (PIP3) signaling, respectively.
Figures
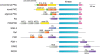


Similar articles
-
The functional relationship of plant lipid-derived second messengers and plant lipid-activated protein kinase.Biochem Soc Trans. 1995 Nov;23(4):871-5. doi: 10.1042/bst0230871. Biochem Soc Trans. 1995. PMID: 8654856 Review. No abstract available.
-
Multiple signaling pathways for the activation of JNK in mast cells: involvement of Bruton's tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7.J Immunol. 1998 Aug 15;161(4):1795-802. J Immunol. 1998. PMID: 9712046
-
Activation of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate.Biochem Biophys Res Commun. 1993 Aug 31;195(1):104-12. doi: 10.1006/bbrc.1993.2016. Biochem Biophys Res Commun. 1993. PMID: 8395820
-
IL-3-induced activation of protein kinases in the mast cell/megakaryocyte R6-XE.4 line.J Immunol. 1990 Mar 1;144(5):1759-66. J Immunol. 1990. PMID: 2307840
-
Following the trail of lipids: Signals initiated by PI3K function at multiple cellular membranes.Sci Signal. 2016 May 17;9(428):re4. doi: 10.1126/scisignal.aad7885. Sci Signal. 2016. PMID: 27188443 Review.
Cited by
-
Protein kinase C pharmacology: refining the toolbox.Biochem J. 2013 Jun 1;452(2):195-209. doi: 10.1042/BJ20130220. Biochem J. 2013. PMID: 23662807 Free PMC article. Review.
-
Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure.J Biol Chem. 2014 Oct 24;289(43):29881-91. doi: 10.1074/jbc.M114.601864. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157099 Free PMC article.
-
The TrkA receptor mediates experimental thermal hyperalgesia produced by nerve growth factor: Modulation by the p75 neurotrophin receptor.Neuroscience. 2017 Jan 6;340:384-397. doi: 10.1016/j.neuroscience.2016.10.064. Epub 2016 Nov 5. Neuroscience. 2017. PMID: 27826102 Free PMC article.
-
Protein kinase C: poised to signal.Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E395-402. doi: 10.1152/ajpendo.00477.2009. Epub 2009 Nov 24. Am J Physiol Endocrinol Metab. 2010. PMID: 19934406 Free PMC article. Review.
-
Cocaine facilitates PKC maturation by upregulating its phosphorylation at the activation loop in rat striatal neurons in vivo.Brain Res. 2012 Jan 30;1435:146-53. doi: 10.1016/j.brainres.2011.11.024. Epub 2011 Nov 13. Brain Res. 2012. PMID: 22208647 Free PMC article.
References
-
- Lemmon M. A. 2008. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9 99–111. - PubMed
-
- Misra S., G. J. Miller, and J. H. Hurley. 2001. Recognizing phosphatidylinositol 3-phosphate. Cell. 107 559–562. - PubMed
-
- Hurley J. H., and T. Meyer. 2001. Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 13 146–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

