The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in 'floral quartet'-like complexes in vitro
- PMID: 19033361
- PMCID: PMC2615621
- DOI: 10.1093/nar/gkn900
The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in 'floral quartet'-like complexes in vitro
Abstract
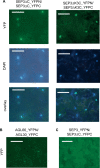
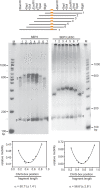
The organs of a eudicot flower are specified by four functional classes, termed class A, B, C and E, of MADS domain transcription factors. The combinatorial formation of tetrameric complexes, so called 'floral quartets', between these classes is widely believed to represent the molecular basis of floral organ identity specification. As constituents of all complexes, the class E floral homeotic proteins are thought to be of critical relevance for the formation of floral quartets. However, experimental support for tetrameric complex formation remains scarce. Here we provide physico-chemical evidence that in vitro homotetramers of the class E floral homeotic protein SEPALLATA3 from Arabidopsis thaliana bind cooperatively to two sequence elements termed 'CArG boxes' in a phase-dependent manner involving DNA looping. We further show that the N-terminal part of SEPALLATA3 lacking K3, a subdomain of the protein-protein interactions mediating K domain, and the C-terminal domain, is sufficient for protein dimerization, but not for tetramer formation and cooperative DNA binding. We hypothesize that the capacity of class E MADS domain proteins to form tetrameric complexes contributes significantly to the formation of floral quartets. Our findings further suggest that the spacing and phasing of CArG boxes are important parameters in the molecular mechanism by which floral homeotic proteins achieve target gene specificity.
Figures




References
-
- Hughes CL, Kaufman TC. Hox genes and the evolution of the arthropod body plan. Evol. Dev. 2002;4:459–499. - PubMed
-
- Veraksa A, Del Campo M, McGinnis W. Developmental patterning genes and their conserved functions: from model organisms to humans. Mol. Genet. Metabol. 2000;69:85–100. - PubMed
-
- Haughn GW, Somerville CR. Genetic control of morphogenesis in Arabidopsis. Dev. Genet. 1988;9:73–89.
-
- Coen ES, Meyerowitz EM. The war of the whorls - genetic interactions controlling flower development. Nature. 1991;353:31–37. - PubMed
-
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature. 1992;360:273–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

