Antitumor effect in medulloblastoma cells by gefitinib: Ectopic HER2 overexpression enhances gefitinib effects in vivo
- PMID: 19033425
- PMCID: PMC2718969
- DOI: 10.1215/15228517-2008-095
Antitumor effect in medulloblastoma cells by gefitinib: Ectopic HER2 overexpression enhances gefitinib effects in vivo
Abstract
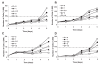
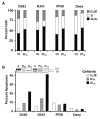
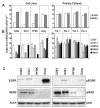
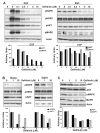
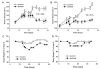
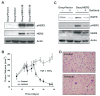
The effects of the epidermal growth factor receptor (EGFR) inhibitor gefitinib on cell growth and signaling were evaluated in three medulloblastoma (MB) cell lines (D283, D341, Daoy), one supratentorial primitive neuroectodermal tumor cell line (PFSK), and four MB primary cultures. Cell lines showed diverse expression of EGFR and human epidermal receptor 2 (HER2), with high levels of constitutively activated HER2 in the HER2-overexpressing D341 and D283 cells. Gefitinib sensitivity varied across lines and was not related to expression of HER receptors or receptor baseline activation. Gefitinib induced G(0)/G(1) arrest in all lines, whereas apoptosis was dose-dependently induced only in D283 and D341 cells. The molecular response to gefitinib was investigated in Daoy and D341 lines, which showed a higher (half-maximal inhibitory concentration [IC(50)], 3.8 microM) and lower (IC(50), 6.6 microM) sensitivity to the agent, respectively. Gefitinib inhibited constitutive and EGF-triggered EGFR phosphorylation in both lines but was ineffective in constitutive activation of HER2 in D341 cells. Phosphorylated AKT inhibition paralleled that of phosphorylated EGFR, suggesting the presence of an autocrine gefitinib-sensitive EGFR/AKT pathway. On the whole, EGF-dependent signaling was less responsive to ligand stimulation and gefitinib inhibition in D341 cells, which correlated with the lower sensitivity to gefitinib's antiproliferative effect of this line. In vivo, the growth of D341 and Daoy xenografts treated with gefitinib at 150 mg/kg per day was inhibited by approximately 50%. Ectopically overexpressed HER2 in Daoy cells significantly increased sensitivity to gefitinib's antitumor effects in vivo (tumor volume inhibition = 78%). Our data indicate that gefitinib might be a molecularly targeted agent for the treatment of MB.
Figures
References
-
- Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. - PubMed
-
- Gilbertson RJ. Medulloblastoma: Signalling a change in treatment. Lancet Oncol. 2004;5:209–218. - PubMed
-
- Luttjeboer M, Kaspers GJ. Medulloblastoma: Need for targeted treatment. Expert Rev Anticancer Ther. 2006;6:649–652. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

