Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets
- PMID: 19033461
- PMCID: PMC2604932
- DOI: 10.1073/pnas.0803488105
Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets
Abstract
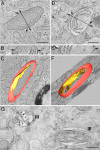
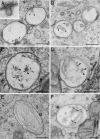
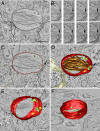
Melanosomes are lysosome-related organelles (LROs) in which melanins are synthesized and stored. Early stage melanosomes are characterized morphologically by intralumenal fibrils upon which melanins are deposited in later stages. The integral membrane protein Pmel17 is a component of the fibrils, can nucleate fibril formation in the absence of other pigment cell-specific proteins, and forms amyloid-like fibrils in vitro. Before fibril formation Pmel17 traffics through multivesicular endosomal compartments, but how these compartments participate in downstream events leading to fibril formation is not fully known. By using high-pressure freezing of MNT-1 melanoma cells and freeze substitution to optimize ultrastructural preservation followed by double tilt 3D electron tomography, we show that the amyloid-like fibrils begin to form in multivesicular compartments, where they radiate from the luminal side of intralumenal membrane vesicles. The fibrils in fully formed stage II premelanosomes organize into sheet-like arrays and exclude the remaining intralumenal vesicles, which are smaller and often in continuity with the limiting membrane. These observations indicate that premelanosome fibrils form in association with intralumenal endosomal membranes. We suggest that similar processes regulate amyloid formation in pathological models.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Seiji M, Fitzpatrick TM, Simpson RT, Birbeck MSC. Chemical composition and terminology of specialized organelles (melanosomes and melanin granules) in mammalian melanocytes. Nature. 1963;197:1082–1084. - PubMed
-
- Chakraborty AK, et al. Polymerization of 5,6-dihydroxyindole-2-carboxylic acid to melanin by the pmel 17/silver locus protein. Eur J Biochem. 1996;236:180–188. - PubMed
-
- Lee ZH, et al. Characterization and subcellular localization of human Pmel 17/silver, a 100-kDa (pre)melanosomal membrane protein associated with 5,6,-dihydroxyindole-2-carboxylic acid (DHICA) converting activity. J Invest Dermatol. 1996;106:605–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

