Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming
- PMID: 19033464
- PMCID: PMC2614796
- DOI: 10.1073/pnas.0810232105
Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming
Abstract
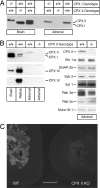
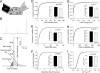
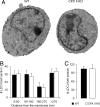
SNARE-mediated exocytosis is a multistage process central to synaptic transmission and hormone release. Complexins (CPXs) are small proteins that bind very rapidly and with a high affinity to the SNARE core complex, where they have been proposed recently to inhibit exocytosis by clamping the complex and inhibiting membrane fusion. However, several other studies also suggest that CPXs are positive regulators of neurotransmitter release. Thus, whether CPXs are positive or negative regulators of exocytosis is not known, much less the stage in the vesicle life cycle at which they function. Here, we systematically dissect the vesicle stages leading up to exocytosis using a knockout-rescue strategy in a mammalian model system. We show that adrenal chromaffin cells from CPX II knockout mice exhibit markedly diminished releasable vesicle pools (comprising the readily and slowly releasable pools), while showing no change in the kinetics of fusion pore dilation or morphological vesicle docking. Overexpression of WT CPX II-but not of SNARE-binding-deficient mutants-restores the size of the the releasable pools in knockout cells, and in WT cells it markedly enlarges them. Our results show that CPXs regulate the size of the primed vesicle pools and have a positive role in Ca(2+)-triggered exocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. - PubMed
-
- Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. - PubMed
-
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. - PubMed
-
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. - PubMed
-
- Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

