Stimulation of neural regeneration in the mouse retina
- PMID: 19033471
- PMCID: PMC2614791
- DOI: 10.1073/pnas.0807453105
Stimulation of neural regeneration in the mouse retina
Abstract
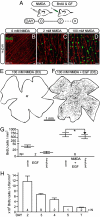
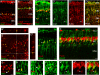
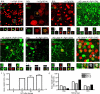
Müller glia can serve as a source of new neurons after retinal damage in both fish and birds. Investigations of regeneration in the mammalian retina in vitro have provided some evidence that Müller glia can proliferate after retinal damage and generate new rods; however, the evidence that this occurs in vivo is not conclusive. We have investigated whether Müller glia have the potential to generate neurons in the mouse retina in vivo by eliminating ganglion and amacrine cells with intraocular NMDA injections and stimulating Müller glial to re-enter the mitotic cycle by treatment with specific growth factors. The proliferating Müller glia dedifferentiate and a subset of these cells differentiated into amacrine cells, as defined by the expression of amacrine cell-specific markers Calretinin, NeuN, Prox1, and GAD67-GFP. These results show for the first time that the mammalian retina has the potential to regenerate inner retinal neurons in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48:1003–1014. - PubMed
-
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23:183–194. - PubMed
-
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24:161–182. - PubMed
-
- Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54:94–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

