Stepwise evolution of protein native structure with electrospray into the gas phase, 10(-12) to 10(2) s
- PMID: 19033474
- PMCID: PMC2587555
- DOI: 10.1073/pnas.0807005105
Stepwise evolution of protein native structure with electrospray into the gas phase, 10(-12) to 10(2) s
Abstract
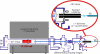
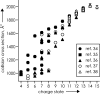
Mass spectrometry (MS) has been revolutionized by electrospray ionization (ESI), which is sufficiently "gentle" to introduce nonvolatile biomolecules such as proteins and nucleic acids (RNA or DNA) into the gas phase without breaking covalent bonds. Although in some cases noncovalent bonding can be maintained sufficiently for ESI/MS characterization of the solution structure of large protein complexes and native enzyme/substrate binding, the new gaseous environment can ultimately cause dramatic structural alterations. The temporal (picoseconds to minutes) evolution of native protein structure during and after transfer into the gas phase, as proposed here based on a variety of studies, can involve side-chain collapse, unfolding, and refolding into new, non-native structures. Control of individual experimental factors allows optimization for specific research objectives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


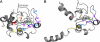


References
-
- Karas M, Bachmann D, Bahr U, Hillenkamp F. Matrix-assisted ultraviolet laser desorption of nonvolatile compounds. Int J Mass Spectrom. 1987;78:53–68.
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. - PubMed
-
- Ganem B, Li Y-T, Henion JD. Detection of noncovalent receptor–ligand complexes by mass spectrometry. J Am Chem Soc. 1991;113:6294–6296.
-
- Ganem B, Li Y-T, Henion JD. Observation of noncovalent enzyme–substrate and enzyme–product complexes by ion spray mass spectrometry. J Am Chem Soc. 1991;113:7818–7819.
-
- Katta V, Chait BT. Observation of the heme–globin complex in native myoglobin by electrospray ionization mass spectrometry. J Am Chem Soc. 1991;113:8534–8535.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

