T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade
- PMID: 19033538
- PMCID: PMC2643911
- DOI: 10.1152/ajpgi.90462.2008
T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade
Abstract
The inflammatory bowel diseases (Crohn's disease; ulcerative colitis) are idiopathic chronic inflammatory disorders of the intestine and/or colon. A major advancement in our understanding of the pathogenesis of these diseases has been the development of mouse models of chronic gut inflammation. One model that has been instrumental in delineating the immunological mechanisms responsible for the induction as well as regulation of intestinal inflammation is the T cell transfer model of chronic colitis. This paper presents a detailed protocol describing the methods used to induce chronic colitis in mice. Special attention is given to the immunological concepts that explain disease pathogenesis in this model, considerations and potential pitfalls in using this model, and finally different "tricks" that we have learned over the past 12 years that have allowed us to develop a more simplified version of this model of experimental IBD.
Figures
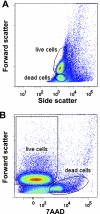
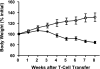
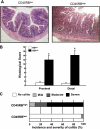


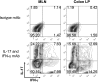
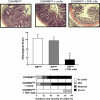
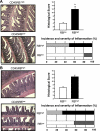



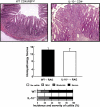
References
-
- Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev 204: 184–194, 2005. - PubMed
-
- Dohi T, Fujihashi K, Koga T, Shirai Y, Kawamura YI, Ejima C, Kato R, Saitoh K, McGhee JR. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology 124: 672–682, 2003. - PubMed
-
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev 206: 260–276, 2005. - PubMed
-
- Fiocchi C Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115: 182–205, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

