Identification of growth processes involved in QTLs for tomato fruit size and composition
- PMID: 19033553
- PMCID: PMC3071768
- DOI: 10.1093/jxb/ern281
Identification of growth processes involved in QTLs for tomato fruit size and composition
Abstract
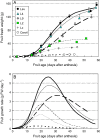
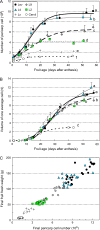
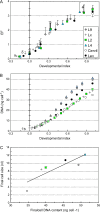
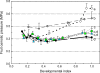
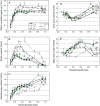
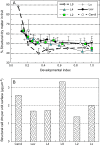
Many quantitative trait loci (QTLs) for quality traits have been located on the tomato genetic map, but introgression of favourable wild alleles into large fruited species is hampered by co-localizations of QTLs with antagonist effects. The aim of this study was to assess the growth processes controlled by the main QTLs for fruit size and composition. Four nearly isogenic lines (NILs) derived from an intraspecific cross between a tasty cherry tomato (Cervil) and a normal-tasting large fruit tomato (Levovil) were studied. The lines carried one (L2, L4, and L9) or five (Lx) introgressions from Cervil on chromosomes 1, 2, 4, and 9. QTLs for fruit size could be mainly associated with cell division processes in L2 and L9, whereas cell expansion was rather homogeneous among the genotypes, except Cervil for which the low expansion rate was attributed to low cell plasticity. The link between endoreduplication and fruit size remained unclear, as cell or fruit sizes were positively correlated with the cell DNA content, but not with the endoreduplication factor. QTLs for fruit composition reflected differences in water accumulation rather than in sugar accumulation, except in L9 for which the up-regulation of sucrose unloading and hexose transport and/or starch synthesis was suggested. This may explain the increased amount of carbon allocated to cell structures in L9, which could be related to a QTL for fruit texture. In Lx, these effects were attenuated, except on fruit size and cell division. Finally, the region on top of chromosome 9 may control size and composition attributes in tomato, by a combination of QTL effects on cell division, cell wall synthesis, and carbon import and metabolism.
Figures

References
-
- Arumuganathan K, Earle E. Estimation of nuclear DNA content of plants by flow cytometry. Plant Molecular Biology Reports. 1991;9:208–218.
-
- Bertin N, Gautier H, Roche C. Number of cells in tomato fruit depending on fruit position and source–sink balance during plant development. Plant Growth Regulation. 2002;36:105–112.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

