Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice
- PMID: 19033663
- PMCID: PMC2582933
- DOI: 10.1172/JCI36843
Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice
Abstract
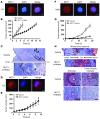
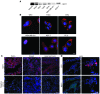
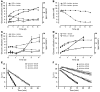
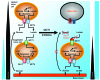
Tumors contain oxygenated and hypoxic regions, so the tumor cell population is heterogeneous. Hypoxic tumor cells primarily use glucose for glycolytic energy production and release lactic acid, creating a lactate gradient that mirrors the oxygen gradient in the tumor. By contrast, oxygenated tumor cells have been thought to primarily use glucose for oxidative energy production. Although lactate is generally considered a waste product, we now show that it is a prominent substrate that fuels the oxidative metabolism of oxygenated tumor cells. There is therefore a symbiosis in which glycolytic and oxidative tumor cells mutually regulate their access to energy metabolites. We identified monocarboxylate transporter 1 (MCT1) as the prominent path for lactate uptake by a human cervix squamous carcinoma cell line that preferentially utilized lactate for oxidative metabolism. Inhibiting MCT1 with alpha-cyano-4-hydroxycinnamate (CHC) or siRNA in these cells induced a switch from lactate-fueled respiration to glycolysis. A similar switch from lactate-fueled respiration to glycolysis by oxygenated tumor cells in both a mouse model of lung carcinoma and xenotransplanted human colorectal adenocarcinoma cells was observed after administration of CHC. This retarded tumor growth, as the hypoxic/glycolytic tumor cells died from glucose starvation, and rendered the remaining cells sensitive to irradiation. As MCT1 was found to be expressed by an array of primary human tumors, we suggest that MCT1 inhibition has clinical antitumor potential.
Figures




Comment in
-
Tumor metabolism: cancer cells give and take lactate.J Clin Invest. 2008 Dec;118(12):3835-7. doi: 10.1172/JCI37373. Epub 2008 Nov 20. J Clin Invest. 2008. PMID: 19033652 Free PMC article.
References
-
- Wu R., Racker E. Regulatory mechanisms in carbohydrate metabolism. IV. Pasteur effect and Crabtree effect in ascites tumor cells. J. Biol. Chem. 1959;234:1036–1041. - PubMed
-
- Dewhirst M.W. Mechanisms underlying hypoxia development in tumors. Adv. Exp. Med. Biol. 2003;510:51–56. - PubMed
-
- Warburg, O. 1930.The metabolism of tumors. Constable. London, United Kingdom. 327 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

