Prostacyclin primes pregnant human myometrium for an enhanced contractile response in parturition
- PMID: 19033666
- PMCID: PMC2582928
- DOI: 10.1172/JCI33800
Prostacyclin primes pregnant human myometrium for an enhanced contractile response in parturition
Abstract
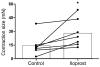
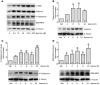
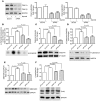
An incomplete understanding of the molecular events that regulate the myometrial transition from the quiescent pregnant state to the active contractile state during labor has hindered the development of improved therapies for preterm labor. During myometrial activation, proteins that prime the smooth muscle for contraction are upregulated, allowing maximal responsiveness to contractile agonists and thereby producing strong phasic contractions. Upregulation of one such protein, COX-2, generates PGs that induce contractions. Intriguingly, the predominant myometrial PG produced just prior to labor is prostacyclin (PGI2), a smooth muscle relaxant. However, here we have shown that activation of PGI2 receptor (IP) upregulated the expression of several contractile proteins and the gap junction protein connexin 43 through cAMP/PKA signaling in human myometrial tissue in organ and cell culture. Functionally, these IP-dependent changes in gene expression promoted an enhanced contractile response to oxytocin in pregnant human myometrial tissue strips, which was inhibited by the IP antagonist RO3244794. Furthermore, contractile protein induction was dependent on the concentration and time of exposure to the PGI2 analog iloprost and was blocked by both RO3244794 and PKA knockdown. We therefore propose that PGI2-mediated upregulation of contractile proteins and connexin 43 is a critical step in myometrial activation, allowing for a maximal contractile response. Our observations have important implications regarding activation of the myometrium prior to the onset of labor.
Figures





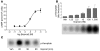


Comment in
-
Possible dual roles for prostacyclin in human pregnancy and labor.J Clin Invest. 2008 Dec;118(12):3829-32. doi: 10.1172/JCI37785. Epub 2008 Nov 20. J Clin Invest. 2008. PMID: 19033650 Free PMC article.
Similar articles
-
Possible dual roles for prostacyclin in human pregnancy and labor.J Clin Invest. 2008 Dec;118(12):3829-32. doi: 10.1172/JCI37785. Epub 2008 Nov 20. J Clin Invest. 2008. PMID: 19033650 Free PMC article.
-
Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells.J Clin Endocrinol Metab. 2009 Feb;94(2):421-7. doi: 10.1210/jc.2008-1723. Epub 2008 Nov 11. J Clin Endocrinol Metab. 2009. PMID: 19001515 Free PMC article.
-
Differential impact of acute and prolonged cAMP agonist exposure on protein kinase A activation and human myometrium contractile activity.J Physiol. 2016 Nov 1;594(21):6369-6393. doi: 10.1113/JP272320. Epub 2016 Aug 8. J Physiol. 2016. PMID: 27328735 Free PMC article.
-
Biochemistry of myometrial contractility.Baillieres Clin Obstet Gynaecol. 1992 Dec;6(4):755-69. doi: 10.1016/s0950-3552(05)80187-7. Baillieres Clin Obstet Gynaecol. 1992. PMID: 1335853 Review.
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
Cited by
-
Myometrial interstitial cells and the coordination of myometrial contractility.J Cell Mol Med. 2009 Oct;13(10):4268-82. doi: 10.1111/j.1582-4934.2009.00894.x. Epub 2009 Sep 2. J Cell Mol Med. 2009. PMID: 19732238 Free PMC article. Review.
-
PKA and AKIP1 interact to mediate cAMP-driven COX-2 expression: A potentially pivotal interaction in preterm and term labour.PLoS One. 2021 Jun 24;16(6):e0252720. doi: 10.1371/journal.pone.0252720. eCollection 2021. PLoS One. 2021. PMID: 34166397 Free PMC article.
-
Uterine injury during diestrus leads to placental and embryonic defects in future pregnancies in mice†.Biol Reprod. 2024 Apr 11;110(4):819-833. doi: 10.1093/biolre/ioae001. Biol Reprod. 2024. PMID: 38206869 Free PMC article.
-
Possible dual roles for prostacyclin in human pregnancy and labor.J Clin Invest. 2008 Dec;118(12):3829-32. doi: 10.1172/JCI37785. Epub 2008 Nov 20. J Clin Invest. 2008. PMID: 19033650 Free PMC article.
-
Urinary prostaglandin metabolites as biomarkers for human labour: Insights into future predictors.PLoS One. 2025 Jul 14;20(7):e0315484. doi: 10.1371/journal.pone.0315484. eCollection 2025. PLoS One. 2025. PMID: 40658667 Free PMC article.
References
-
- Wolfe C., Poston L., Jones M. Digoxin-like immunoreactive factor, corticotropin-releasing factor, and pregnancy. Lancet. 1987;1:335–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

