Characterization of alkaline transitions in ferricytochrome c using carbon-deuterium infrared probes
- PMID: 19035653
- PMCID: PMC2853379
- DOI: 10.1021/bi801223n
Characterization of alkaline transitions in ferricytochrome c using carbon-deuterium infrared probes
Abstract
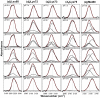
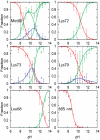
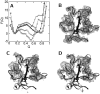
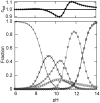
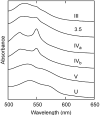
The alkaline-induced structural transitions of ferricytochrome c have been studied intensively as a model for how changes in metal ligation contribute to protein function and folding. Previous studies have demonstrated that multiple non-native species accumulate with increasing pH. Here, we used a combination of experiments and simulations to provide a high-resolution view of the changes associated with increasing alkaline conditions. Alkaline-induced transitions were characterized under equilibrium conditions by following changes in the IR absorptions of carbon-deuterium chromophores incorporated at Leu68, Lys72, Lys73, Lys79, and Met80. The data suggest that at least four intermediates are formed as the pH is increased prior to complete unfolding of the protein. The first alkaline transition observed appears to be driven by a single deprotonation and occurs with a midpoint of pH 8.8, but surprisingly, the intermediate formed does not appear to be one of the well-characterized lysine misligates. At higher pH, second and third deprotonations, with a combined apparent midpoint pH of 10.2, induce transitions to Lys73- or Lys79-misligated species. Interestingly, the lysine misligates appear to undergo iron reduction by the coordinated amine. A transition from the lysine misligates to another intermediate, likely a hydroxide-misligated species, is associated with a fourth deprotonation and a midpoint of pH 10.7. Finally, the protein loses tertiary structure with a fifth deprotonation that occurs with a midpoint of pH 12.7. Native topology-based models with enforced misligation are employed to help understand the structures of the observed intermediates.
Figures
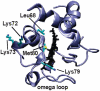




References
-
- McLachlan AD. Rapid comparison of protein structures. Acta Crystallogr. 1982;A38:871–873.
-
- Scott RA, Mauk AG. University Science Books; Sausalito, CA: 1996. pp. 611–634.
-
- Theorell H, Akesson AJ. Studies on cytochrome c. II. The optical properties of pure cytochrome c and some of its derivatives. J. Am. Chem. Soc. 1941;63:1812–1818.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

